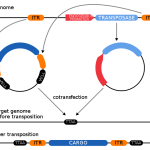
Over millennia, there has been a seamless continuum of technologies for genetic modification of plants, animals, and microorganisms, with progressive improvements in precision and predictability – a fact that seems to have escaped the notice of EU politicians and regulators.
regulation
Artificial Intelligence plays an increasingly prominent role in modern life, medicine included. While the technology promises to improve health care in many ways, it also carries potentially serious risks. That raises a critical question: when AI harms patients, who's responsible?
Here's an article I co-authored with Emily Hemendinger for The Conversation. You can find the original version at the link at the bottom of the article.
A teenager died recently after taking the "One Chip Challenge," eating Paqui's uber-spicy tortilla chip and going as long as possible without eating or drinking anything else. The cause of death remains unclear, but there's an interesting lesson here about the uselessness of "non-GMO" food labels. Meanwhile, actress Jessica Biel sells all-natural Tylenol — which is identical to plain ole' Tylenol. Another case of dubious health marketing? Yep.
Americans need assurance of the availability of approved drugs and medical devices in the marketplace so that healthcare providers have more reliable inventory, experience fewer shortages, and more choices when shortages arise. Reciprocity of regulatory decisions would help to achieve that.
For decades, excessive, unscientific regulation has slowed innovation using molecular genetic engineering. Policymakers must awaken to the realization that regulations based on pseudoscience or nescience are destructive and regressive. Tremendous innovations await, if only we have the wisdom to permit them to be developed.
The agency's primary functions are ensuring food safety, regulating tobacco products rationally, and expeditiously approving new drugs and medical devices. It's failing. Instead, we're getting increasingly complex organizational structures and the commissioning of endless reports.
A concerning shortage of Adderall, one of the drugs commonly used to treat attention deficit hyperactivity disorder (ADHD), is putting patients at risk. What caused it, and how can we fix it? The EPA has set new guidelines to keep PFAS out of drinking water. There's a problem, however: the agency's standards are absurd.
Panicked headlines recently warned that the popular artificial sweetener erythritol could increase heart disease risk. The study that generated these claims in no way supports that association. Dietary supplements are a multi-billion-dollar industry; they've also killed people. Do they need more regulatory oversight?
In 2016, the American viewing public was exposed to 663,000 television commercials for pharmaceuticals. That is a significant “ad spend” by Pharma, which we pay for through increased drug pricing. A new study looks at the therapeutic value of the more heavily advertised drugs. The key concept here is “market differentiation.”
The FDA actively – and unusually – collaborated with a biopharmaceutical company to pursue the approval of an Alzheimer's Disease drug, despite many questions about its efficacy and astronomical price. A congressional investigation found that the FDA’s approval process was “rife with irregularities" and that the agency’s actions “raise serious concerns about FDA’s lapses in protocol.”
It appears to defy logic that naloxone, the antidote for opioid overdoses, isn't available on demand. After all, it's a lifesaving drug with no potential for abuse. But it's not so simple, as Dr. Jeffrey Singer explains.