
Nothing new here. We ve written about this before.
Once the esteemed New York Times columnist Nick Kristof gets out of his comfort (and knowledge) zone he goes from a really great commentator to an ignorant scaremonger. Sort of a Dr. Jekyll and Mr. Hydrogen.
But even he has outdone himself this time. In an effort to be seen as a superhero against the evil chemical industry he is grabbing for straws that don t even exist.
In a scientifically amateurish attempt to make a ridiculous point, he only succeeds in demonstrating (at least to anyone who has taken a chemistry course) how far he is willing to go to find a villain. But it is what he does not say that reveals how little sense his arguments actually make.
Kristof is now equating common chemicals, that have never been proven to harm any human to a real poison, which was commonly used long ago lead. This is like comparing a mousetrap to a beartrap.
The essence of Kristof s argument is that there is somehow a similarity between the use of huge amounts of lead a known, serious toxin to a group of chemicals that we have all been exposed to for decades, and resemble lead as much as they resemble Key Lime Pie.
First, let s take a look at why lead was used in the first place. Its use in gasoline was to prevent engine knock. It has successfully (and rightfully so) been replaced by other chemicals that do the same job. Getting rid of lead in gasoline was by any measure a very important step in protecting human health and the environment.
Its use in paint was no accident either. It was used as a pigment for color, durability and opacity. Except that by an unfortunate coincidence, lead paint has a sweet taste, which led many children to eat it, some of whom suffered serious neurological problems. Like leaded gasoline, it had to go. And it has been banned in the U.S. from virtually all products since 1978. Since then, it has been replaced by titanium dioxide, which is as close to non-toxic as a chemical can get.
ACSH s Dr. Bloom, who wrote a 2012 Manhattan Institute op-ed entitled Why I Don't Write About Pottery From the Ming Dynasty commented about Kristof s latest perilous journey into science. He says, When in doubt, use the scary, but meaningless term endocrine disruptor, and you ll grab some attention every time. Especially when there s nothing real to talk about.
To try to explain an essentially meaningless term, endocrine disruptor seems to encompass any chemical that bears any chemical resemblance to human hormones. From there, it is a giant leap to conclude that this in itself constitutes a risk.
Dr. Bloom explains, human sex hormones bind to various receptors within the body, producing a myriad of physiological effects all necessary for normal function. This is how our bodies work. But where proponents of the endocrine disruptor theory fall on their scientific faces is when they fail to mention the ability of these substances to bind to human sex hormone receptors. And it is here where their reasoning breaks down.
As illustrated in the graphic below, two of the perennial villains, BPA and methyl paraben do bind to estrogen receptors but do so ten-to-hundreds of times more weakly than natural estrogens. This means that a receptor meant to bind to estrogens is going to be bound to estrogens not BPA or parabens.
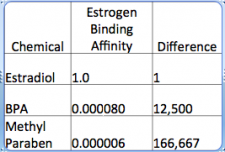
Dr. Bloom adds, Kristof s attempt to equate real, deadly toxins with so-called 'endocrine disruptors' is pure scientific craziness. Yet, people will believe him. Academic careers are built, and environmental groups stay funded by trying to find something wrong real or imagined with everyday chemicals. Such is the sad state of science in the world today."


