 Normally, this wouldn t even make the news. A new antibiotic approved. Not only that, but it belongs to a class of antibiotics (called cephalosporins) from the class of 1960s, which is hardly novel. There are about 60 cephalosporins that have been approved since 1964, when cephalothin was launched by Lilly.
Normally, this wouldn t even make the news. A new antibiotic approved. Not only that, but it belongs to a class of antibiotics (called cephalosporins) from the class of 1960s, which is hardly novel. There are about 60 cephalosporins that have been approved since 1964, when cephalothin was launched by Lilly.
In today's just because department, you might find it interesting to know how cephalosporins were discovered in the first place: from a sewer in Sardinia in 1948, proving that 1) You never know what you will find and where; 2) Be thankful for your job.
Yet, today s FDA approval of Cubists Zerbaxa, which is actually a combination of the actual antibiotic (ceftolozane) plus tazobactam a commonly used additive to antibiotics that partially protects them from being converted by the bacteria into a non-active metabolite.
What makes cephalosporin #61 so special is that most of the others no longer work against a variety of infections, such as the now-infamous MRSA. (Note that not all cephalosporins are useless. Some are used quite successfully against certain infections, however, resistance will eventually become a problem here as well.)
ACSH s Dr. Josh Bloom s 2012 New York Post op-ed entitled The Coming Gonorrhea Epidemic spoke about how scary the crisis of antibiotic resistance really is: Gonorrhea is becoming untreatable. This common and potentially serious sexually transmitted disease was once easily cured. But now, of the 50 antibiotics once used to treat the infection, only one drug works and just barely.
The drug in question, ceftriaxone, also a cephalosporin, was all that was left to treat a sexually transmitted disease that killed people in the 12th century. And in 2012, the FDA restricted its use to injection only. This is helpful in slowing down resistance, since one of its contributing causes is patients not taking all of the pills.
So, why is this story much bigger than it would have been years ago? The chart below should answer that.
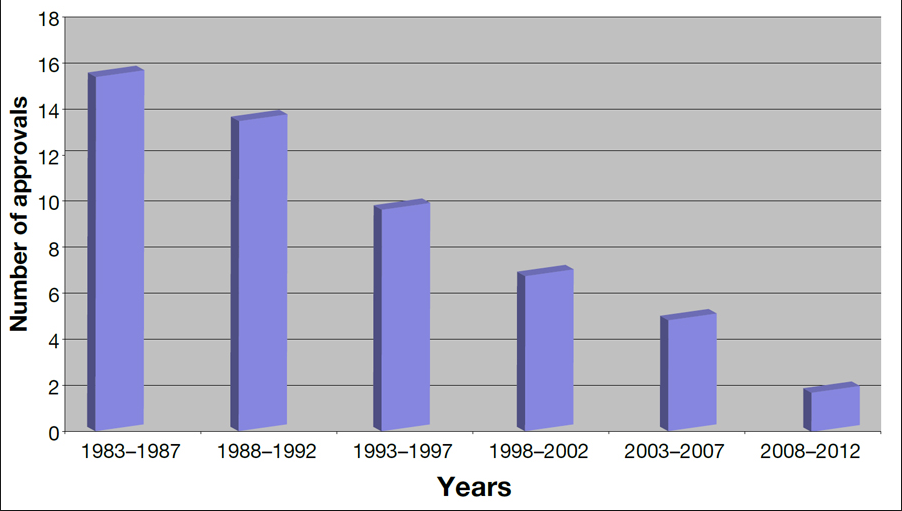
Not only, have bacteria learned new ways to defeat antibiotics, but the number of new ones has dropped dramatically, mostly because of new, onerous clinical trial requirements that were established by the FDA in the 1990s (for no good reason whatsoever). The new requirement roughly doubles the number of enrollees needed for a trial, making it prohibitively expensive and sometimes logistically impossible. Drug companies dropped out of this area of research, which is one of the two principal reasons we are in this mess now.
But, the news is getting better. Thanks to the FDA, which finally relaxed (and then some) its rules for antibiotic development, and a few companies that took some chances, things are turning around.
ACSH advisor Dr. David Shlaes, one of the top antibiotic experts you ll ever find (we often refer to his blog Antibiotics-the Perfect Storm), who was instrumental in talking some sense into the FDA, is optimistic, perhaps for the first time in memory.
He says, Zerbaxa (cetolozane-tazobactam) is the fourth new antibiotic to be approved by the FDA this year alone. This is about the same number approved between 2005 and 2012! In addition, the FDA advisory committee just voted for approval of cetazidime-avibactam - so it is likely to be approved early next year.
Speaking of the FDA, Shlaes explains: The latter approval was based on the FDA s new unmet needs guidance and used primarily phase 2 clinical data to justify the approval. This is a historic development for the FDA. All this means that the FDA has gotten serious about providing a robust pipeline of antibiotics active against resistant pathogens - something new for them since 2012. This can be nothing but good news for society globally. Antibiotics are back!
Dr. Bloom says, Every once in awhile, our government actually does something right. As Dr. Shlaes mentioned, the FDA has done a 180. Instead of requiring huge phase III trials, they are being much more proactive and really working with companies to get us out of the trouble we are in. Good for them and for us.


