The Frank R. Lautenberg Chemical Safety for the 21st Century Act amends the Toxic Substances Control Act (TSCA) and was signed into law June 22, 2016. It created a mandatory requirement for EPA to evaluate existing chemicals with clear and enforceable deadlines, to do so in a transparent fashion, and to do so using risk-based chemical assessments rather than rely on simple epidemiological correlations.
EPA selected the first 10 chemicals to undergo risk evaluation under the amended TSCA and to make those understandable for the public, the American Council on Science and Health is producing risk-based evaluations of each, which will then be compiled into a free downloadable book for consumers and policy makers.
What is Carbon Tetrachloride?
Carbon tetrachloride is also known as tetrachloromethane and perchloromethane. It is a colorless liquid with a sweet odor that evaporates easily but does not readily burn. Most carbon tetrachloride that escapes to the environment is found as a very stable gas, which can be smelled by people in concentrations as low as 10 parts per million (ppm), according to the US Agency for Toxic Substances and Disease Registry (ATSDR, 2005) and US Environmental Protection Agency (EPA, 2010).
Carbon tetrachloride has been widely used in industry and dry-cleaning establishments as a degreasing agent, and in households as a spot remover for clothing, furniture, and carpeting. It was also used in fire extinguishers, as a fumigant to kill insects in grain, and as a pesticide. Carbon tetrachloride has been produced in large quantities to make refrigeration fluid and propellants for aerosol cans. It is a solvent for oils, fats, lacquers, varnishes, rubber waxes, and resins, and a starting material in the manufacturing of organic compounds. Consumer and fumigant uses of carbon tetrachloride have been discontinued and only industrial uses remain. Carbon tetrachloride does not occur naturally in the environment.
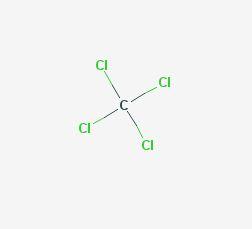
The chemical structure of carbon tetrachloride is very simple as shown at the US National Library of Medicine’s Toxnet database (NLM, 2018a). Carbon tetrachloride belongs to the class of organic compounds known as halomethanes and is made by substituting four chlorine atoms for the four hydrogen atoms on the methane molecule (formula CCl4). Exposure to high levels by inhalation or ingestion can affect the central nervous system, liver, kidneys, and lungs in humans. However, high levels in animals have resulted in modest toxicity from inhalation exposure, low-to-moderate toxicity from ingestion, and moderate toxicity from dermal exposure (ATSDR, 2005; EPA, 2010).
Exposure To Carbon Tetrachloride
Most carbon tetrachloride released to the environment is expected to volatilize rapidly due to its high vapor pressure. Most carbon dioxide releases to the environment are from industrial processes or old bottles of cleaning agents containing carbon tetrachloride that may still be in the home. Carbon tetrachloride does not bind strongly to the soil and dust particles and, thus, is expected to be highly mobile in soil and groundwater. In the environment, carbon dioxide is broken down slowly and may accumulation in the atmosphere as well as the groundwater. Although carbon tetrachloride is widely dispersed and persistent in the environment, it is not detected frequently in foods.
Inhalation of carbon tetrachloride appears to be the major route of exposure for workers and also for the general population in the United States (ATSDR, 2005). The reported average concentrations of carbon tetrachloride in air in several areas of the United States ranged between 0.6 and 1.0 microgram per cubic meter (μg/m3), which is equivalent to 0.1–0.16 parts per billion (ppb). Also, in several homes in U.S. cities, typical indoor concentrations were about 1.0 μg/m3 (0.16 ppb), with some values up to 9 μg/m3 (1.4 ppb). According to ATSDR, concentrations of carbon tetrachloride in indoor air are expected to be higher than in outdoor air because of the presence of carbon tetrachloride in building materials or household products.
The general population is also exposed to carbon tetrachloride by ingestion of contaminated drinking water. However, in a majority of domestic water supplies in the U.S, concentrations of carbon tetrachloride were below 0.5 μg/liter (μg/L). It has been estimated that the average daily intake of carbon tetrachloride for the general population is 0.1 microgram per kilogram body weight per day (μg/kg-day) from inhalation exposure and 0.01 μg/kg-day from ingesting drinking water containing typical low concentrations of the chemical.
Individuals may be exposed to carbon tetrachloride in the air from accidental releases from production and uses, from its disposal in landfills where it may evaporate into the air or leach into groundwater, and from volatilization of contaminated water during showering or bathing. However, the general population is not likely to be exposed to large amounts of carbon tetrachloride in this fashion. In contrast, populations living within or very near waste sites, or areas of heavy carbon tetrachloride use might have an increased risk of exposure from contaminated media (air, water, or soil).
In addition, people may also be exposed to carbon tetrachloride from building materials or products, such as cleaning agents, used in the home. Furthermore, those likely to receive the highest levels of exposure are those who are involved in the production, formulation, handling, and application of carbon tetrachloride, or workers directly involved in the manufacture or use of carbon tetrachloride.
Carbon Tetrachloride Health Effects
Carbon tetrachloride is readily absorbed by all routes of exposure (inhalation, oral and dermal) in both humans and experimental animals. Once absorbed it is widely found in fat but also in blood, muscle, liver, brain and other tissues and organs. The absorbed carbon tetrachloride is metabolized via several pathways in either the presence or absence of oxygen. Metabolism of the absorbed carbon tetrachloride results in the a number of metabolites, some of which may directly bind to proteins and DNA, or degrade lipids in the cell membranes, or alter calcium levels in the cell. The toxic effects of carbon tetrachloride are generally attributed to these reactive products. When exposed to carbon tetrachloride, humans and animals will also excrete the unmetabolized parent compound in exhaled air.
Like exposure to any chemical, toxicity of carbon tetrachloride depends on the level to which one is exposed and the length of time of exposure. Both ATSDR and EPA have an abundance of health effects information on carbon tetrachloride. Studies in experimental animals, combined with limited observations in humans, indicate that the principal adverse health effects associated with inhalation exposure to carbon tetrachloride are central nervous system (CNS) depression, liver damage, and kidney damage.
Humans exposed briefly to carbon tetrachloride though inhalation or ingestion experienced effects such as headache, dizziness, and weakness. At higher exposures, tremor, blurred vision, drowsiness, seizures, and loss of consciousness as well as nausea, vomiting, diarrhea, and abdominal pain and/or damage to the liver and kidney may occur. In some case reports, a high intake of alcohol resulted in enhanced carbon tetrachloride toxicity following inhalation exposure.
Longer-term occupational exposures of carbon tetrachloride also resulted in GI effects, liver and kidney effects, headache, and dizziness. Although epidemiological investigations have associated oral carbon tetrachloride exposure with a variety of birth defects, it is unclear whether these effects were due to carbon tetrachloride, since exposures to other chemicals were involved and such studies were considered limited. There is no evidence for reproductive or developmental toxicity in humans exposed by inhalation to carbon tetrachloride. Epidemiological studies have investigated potential associations between cancer of various types and exposure to carbon tetrachloride.
However, there is no available evidence that carbon tetrachloride causes any tumors in humans, contrary to the evidence in experimental animals (EPA, 2010).
CNS and liver effects occur in experimental animals following single, high inhalation exposures, similar to those observed in humans (ATSDR, 2001; EPA, 2010). Liver effects, and to a lesser extent kidney effects, appear to be the primary effects after short-term duration inhalation exposures. Long-term inhalation exposure to carbon tetrachloride also resulted in effects on the liver, kidney, and spleen as well as effects on the testis, decreased growth, and reduced survival. In high dose, oral studies, the liver appears to be the primary target organ, with damage to the kidney occurring at slightly higher doses.
The liver is also the most prominent target of carbon tetrachloride in shorter- and longer-term oral studies of experimental animals. Both oral and inhalation exposures have resulted in hepatic tumors in multiple experimental animal species and adrenal gland tumors in a single species (EPA, 2010).
Carbon TetrachlorideSafe or Virtually Safe Levels
The federal and state governments develop regulations and recommendations to protect public health. Regulations and recommendations are often expressed as a safe or virtually safe level, that is, a level of a substance in air, water, soil, or food that is not expected to cause any adverse health effect, even in people who are sensitive to the chemical’s effects. These safe levels are usually based on information from experiments with animals (usually rodents) at much higher levels of the chemicals than humans would typically encounter. The higher animal exposures are used to see what adverse health effects occur, and scientists then conjecture what the adverse effects could possibly be in humans at a lower level of exposure. Scientists can then estimate the level that will very likely protect humans. Sometimes these safe levels differ among federal and state organizations because they used different assumptions for human exposure, different animal studies, or employ methods that differ slightly. Other times these recommendations differ because new science develops that suggests different levels are toxic or safe. ATSDR (2001) and NLM (2018b) give examples of these differing recommendations for carbon tetrachloride among government organizations. Recommendations and regulations are also updated periodically as more information becomes available.
ATSDR (2005) has derived safe or virtually safe levels (referred to as minimal risk levels or MRLs for carbon tetrachloride. (Minimal Risk Level as an estimate of daily human exposure to a substance that is likely to be without an appreciable risk of adverse effects (noncarcinogenic) over a specified duration of exposure. MRLs can be derived for acute, intermediate, and chronic duration exposures for inhalation and oral routes. No MRL was established for 14 day or less inhalation exposure. An MRL was set at 0.03 parts per million (ppm) (or 0.20 mg/m3) for either short term or longer-term exposures based on liver effects. For the oral route, MRLs of 0.02 milligrams per kilogram body weight per day (mg/kg-day) and 0.007 mg/kg-day have been derived, based on liver toxicity, for the 14 day or less exposure and intermediate exposures to carbon tetrachloride, respectively. No chronic MRLs have been developed for carbon tetrachloride.
The long-term safe levels for non-cancer toxicity derived by EPA are referred to as the reference concentration or dose (RfC or RfD for the inhalation and oral routes, respectively.) Reference Concentration (RfC) or Reference Dose (RfD) is an estimate of the chemical concentration or dose that will not cause non-cancer effects during a specified exposure period.The EPA (2010) RfC and RfD for carbon tetrachloride are 0.1 mg/m3 and 0.004 mg/kg-day, respectively. For cancer, EPA estimated a risk 6 × 10-6 (0.6 persons in 100,000 folks) per μg/m3 for adrenal gland tumors. The oral cancer risk was 7 × 10-5 (7 persons in 100,000 folks) per ug/kg-day based on liver tumors.
All of these values can be viewed at the National Library of Medicine’s Toxnet (NLM, 2018b)
Why Is EPA Looking At This Under The Lautenberg Chemical Safety Act?
EPA scientists are currently looking at the likely routes of exposure to carbon tetrachloride in the environment and will be developing exposure scenarios, or pathways, of how the public comes into contact with it. These exposure pathways will then be studied by EPA scientists by comparing the amount of carbon tetrachloride exposure in the pathway to its safe or virtually safe level.
If human exposure in the pathway is at or below this safe or virtually safe level, then carbon tetrachloride exposure from the pathway is not considered to be a human health concern. If exposure is above this safe or virtually safe level, then the pathway might be considered as a possible health concern; several pathways may be added together to suggest a health concern. Small excesses of the safe or virtually safe dose are seldom cause for concern since these safety levels are developed from conservative assumptions, including the use of safety factors that tend to exaggerate risk and exposure pathways that tend to exaggerate exposure.
In either event, regulations might be developed to lessen the exposure of carbon tetrachloride from this pathway(s). See also EPA (2018) for specific questions related to the assessment of carbon tetrachloride under the new LCSA.
Controversy Over Carbon Tetrachloride
EPA (2018) interprets the mandates within the LCSA to conduct risk evaluations on current and prospective uses of carbon tetrachloride for which manufacturing, processing, or distribution in commerce “is intended, known or reasonably foreseen.” Carbon tetrachloride has a number of uses in these categories that are included in the current scope of EPA’s LCSA evaluation. However, EPA is excluding from its problem formulation any exposure pathways to human or ecological receptors from environmental releases and waste streams associated with industrial and commercial activities for carbon tetrachloride that are already under programs of other environmental statutes administered by EPA. These other programs adequately assess and effectively manage exposures of carbon tetrachloride, some of which have long-standing regulatory and analytical processes. These pathways include the ambient air pathway under the Clean Air Act, the drinking water pathway under the Safe Drinking Water Act, the ambient water and the biosolids pathways under the Clean Water Act, and the regulation of hazardous wastes under the Resource Conservation and Recovery Act. Each of these exclusions may cause some controversy.
In addition, EPA (2010) has concluded that carbon tetrachloride is “likely to be carcinogenic to humans” by all routes of exposure, despite inadequate evidence of in humans, because sufficient evidence exists in experimental animals by oral and inhalation exposure. The International Programme on Chemical Safety (IARC, 1999) has also concluded overall that carbon tetrachloride is possibly carcinogenic to humans (Group 2B).
Although these statements are not controversial, EPA (2010) has indicated that cancer is more likely formed indirectly rather than by direct mutation. The liver carcinogenicity, according to EPA, occurs at carbon tetrachloride exposures that also induce toxicity to the liver cells and a sustained regenerative and proliferative response; exposures that do not cause hepatotoxicity are not expected to result in liver cancer.
Despite this statement, EPA applied a low-dose linear extrapolation approach to the quantitative evaluation of carbon tetrachloride carcinogenicity. The application of this conservative linear model has also generated some controversy.
More analyses in our series on the Lautenberg Chemical Safety Act compounds:
ACSH Explains: What's The Story On Bromopropane?
ACSH Explains: What's The Story On Dioxane?
ACSH Explains: What's The Story On Trichloroethylene (TCE)?
ACSH Explains: What's The Story On Methylene Chloride (DCM)?
ACSH Explains: What's The Story On Asbestos?
REFERENCES:
International Programme on Chemical Safety (IPCS) (1999). Carbon Tetrachloride. Environmental Health Criteria 208. WHO. Geneva. Available at: http://inchem.org/documents/ehc/ehc/ehc208.htm.
U.S. Agency for Toxic Substances and Disease Registry (ATSDR). 2005. Toxicological Profile for Carbon tetrachloride. August. Available at: https://www.atsdr.cdc.gov/toxprofiles/tp30.pdf
U.S. Environmental Protection Agency. 2010. Toxicological Review of Dichloromethane (Carbon tetrachloride). Integrated Risk Information System (IRIS). National Center for Environmental Assessment. EPA/635/R-08/005F. Washington D.C. available at: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0020tr.pdf.
U.S. Environmental Protection Agency. 2018. Problem Formulation of the Risk Evaluation for Carbon Tetrachloride (Methane, Tetrachloro-). Office of Chemical Safety and Pollution Prevention. EPA Document# EPA 740-R1-7016. Available at https://www.epa.gov/sites/production/files/2018-06/documents/ccl4_problem_formulation_05-31-18.pdf
U.S. National Library of Medicine. 2018a. ChemIDplus: Carbon tetrachloride. Toxnet database. Found at: https://chem.nlm.nih.gov/chemidplus/name/carbon%20tetrachloride
U.S. National Library of Medicine. 2018b. International Toxicity Estimates for Risk (ITER) database. Carbon tetrachloride. Found at: https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/f?./temp/~H9qlWr:7.
Written by
Bernard Gadagbui, MS, PhD, DABT, ERT
Bethany Hansen, MA
Michael Dourson, PhD, DABT, FATS, FSRA
all of Toxicology Excellence for Risk Assessment (TERA)