In the world of simulations, some things are relatively easy, In engineering, every graduate has written a computer program that may create a better widget, but if it has to be machined to 1.01236568 inches, it isn't much help in actual construction. If you have ever read the preprint site arXiv, you've seen mathematicians show how time travel should be possible. Math is a language and in language lots of things are possible. Physics are another issue.
So it goes with drug development. As Dr. Josh Bloom, the Council's Senior Director of Chemical and Pharmaceutical Sciences, with over three decades of practical experience says, "On a computer, every disease has been cured 10,000 times."
So while media may be excited about a claim that combining four or five existing products could help slow antibiotic-resistant bacteria, scientists in the real world are more skeptical. Combining two might make sense but they still have to go through trials first, and that could quickly mean diminishing returns, while three would be susceptible to interactions you wouldn't know about before clinical trials. But in a very narrow simulation - eight antibiotics and E. coli,18,278 combinations - the academic scholars declare they were "startled by how many potent combinations they discovered."
Huzzah, all those corporate scientists can learn a thing or two from the Systems Biology and Applications journal, right? Not at all, that is what you do your first month on the job in a drug company, so no chemist is "startled" when it happens. Creating a miracle drug is part of the learning process, to get the butterflies out of your system and learn how the world really works. Over time every drug development group predicts how well a compound, or in this case drug combination, might work. Then they do what they can to simulate it as accurately as possible. But there is a reason that for every 5,000 compounds that survive an academic part of development, perhaps one will make it to market. And that most researchers will never have a successful drug in their careers. It isn't for lack of knowing how to simulate on a computer, it's that a lot of things work on a computer.
What is really important occurs after the simulations are done, and that is why it's important that more journalists read beyond the press release.(1)
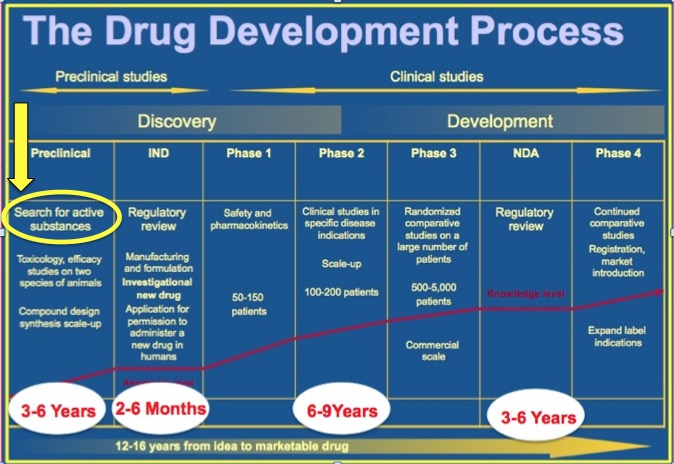
It's not a knock on the paper authors, they need to get their work out there and "startled" is the go-to cliché to get media attention so it was probably a university press liason writing it. It's great that 8,000 combinations might work on a computer, but that's still only the start. Next, scientists would have to agree they are practical and not that widget in paragraph one, then they would have to go into animal studies. Whatever still seems to work in animals would go into a small clinical trial (Phase I), with a dozen or more people, to establish safety and dosing. If it is safe, a Phase II trial would establish that it works in humans even though it worked on a computer and in rodents. That's another two years or so. If it passes Phase II, it has to show in Phase III that it works well enough to be a benefit compared to other treatments. Finally, a Phase IV trial would involve thousands of participants.You can imagine why it costs an average of $1.5 billion to get a product to market, and why there is a chasm between what someone might find in a computer simulation and what people spending money believe about reality.
Courtesy of Steve Schow, Ph.D.
Antibiotics are also a different market than other drugs. They are not chronic products, like for cancer, heart disease or musculoskeletal conditions, they are used for such a short period of time it is difficult to make a profit if the development costs are ridiculous. And they can be ridiculous. In the past statisticians in the FDA infectious disease advisory committee created absurd (bordering on impossible) requirements for clinical studies of antibiotics. Since they set out to do nothing but improve the statistical power of the trials they were prohibitively expensive and clinically meaningless. The result: the best companies abandoned the market.
Current FDA Commissioner Scott Gottlieb, M.D., has undone a lot of the mistakes made by previous administrations but statisticians in government are often career employees and created those policies because they believe in statistics and they want to translate that into trials. Such culture is not easy to change, we won't convince epidemiologists that statistics don't lead to better drugs any more than we can convince mathematicians that time travel isn't possible. Yet the process must be improved if we want the best scientists working on these issues. Sorry, haters, the best drug chemists means drug companies. Antibiotic resistance is inevitable, but responding to it takes people who are brilliant, and that means they have to get paid.
As Dr. Bloom said in 2013, "Anyone who thinks that this can be solved by government research hasn’t the wildest idea of the Sisyphean task of drug development" and "possibly the only way to deal with this would be major government financial incentives either through outright research subsidies or vastly extended patent coverage to drug companies something that is most likely politically impossible given the misdirected hatred toward drug companies."
Hatred that has only gotten worse since then.
NOTE:
(1) For decades, we have published a media guide for science journalists but it is relevant to anyone who cares about critical thinking, especially at a time when far too many journalist are so interested in being against corporations they will embrace anything from universities.




