Now and then I write about some of the atrocities that I used to work with (or avoid) (See Butyllithium — Something You Really Don't Want To Mess With) or that others used and paid a huge price (See Two Drops Of Death: Dimethylmercury) (1,2).
Since some of you sadists out there seem to enjoy these, and I'm sick of writing about kale, it's time for another chemical from hell. For all of you scaredy-cats who are terrified of Diet Coke, cash register receipts, or Tupperware containers, I invite you to sit back while I regale you with stories of a real man's chemical - methyl fluorosulfonate aka "Magic Methyl," a molecular baddass if ever there were one (Figure 1).

Figure 1. Methyl groups (red circle) are ubiquitous and normally benign. But when they are bonded to a "deathyl group" (aka fluorosulfonate, blue circle) the combination makes an extremely deadly toxin - "badasses "Magic Methyl," a chemical to be feared by everyone. Even Clint. Photo: Marriage Celebrant
HOW DOES IT WORK?
As the name implies, Magic Methyl is a methylating agent - a chemical that reacts with certain molecules or biomolecules (e.g., amino acids, DNA) to replace a hydrogen atom with a methyl group. Methylating agents cause damage to DNA, which makes these chemicals mutagenic and possibly carcinogenic. Inhibition of DNA methylation has been studied as a method to treat cancer.
Even though prolonged exposure to Magic Methyl would probably give you cancer, this is the least of your worries. But this isn't:

Source: J. Admiraal The Lancet, 308(7990), 854 (1976).
WHY IS MAGIC METHYL SO TOXIC?
The fluorosulfonate portion of the molecule is responsible for turning a simple methyl group into a stone-cold killer. To understand this, you need an episode of... The Dreaded Chemistry Lesson From Hell.©® (Figure 2)
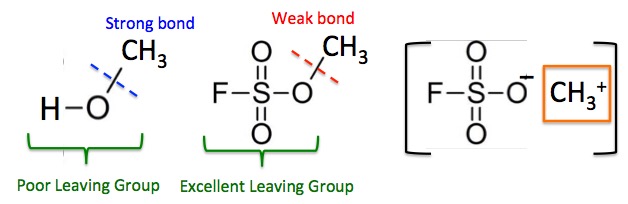
Figure 2. The relative strength of methylating agents is highly dependent on what is attached to the methyl group.
The carbon-oxygen bond in methanol (left) is strong; it is very difficult to break and this makes methanol a poor methylating agent. The bond is so strong because breaking it requires that the hydroxide (OH-) anion is expelled - a high energy process. Hydroxide is a poor leaving group because of this. But in Magic Methyl (center) the fluorosulfonate part of the molecule is an excellent leaving group, which makes the C-O bond very weak and the methyl group extremely reactive, so Magic Methyl is a very strong methylating agent. Although the chemical is not ionized as shown in brackets on the right, the methyl group has characteristics (orange box) of the methyl cation (CH3+), which is so reactive that it will methylate nitrogen, oxygen, and sulfur atoms, a box of Wheat Thins or your mother-in-law. This is why Magic Methyl is so chemically reactive.
BUT IT IS ALSO DANGEROUS FOR ANOTHER REASON
Magic Methyl also breaks down (just because it feels like it) to give a Satanic chemical called fluorosulfonic acid, which is such a strong acid that it eats skin, causing very severe burns.

Fluorosulfonic acid dissolving a chicken breast. Still better than Chipotle. Source: YouTube
And if that isn't bad enough, it also decomposes violently in water to give hydrogen fluoride (eats glass) and sulfuric acid (spews from volcanoes). So, it's not surprising that Magic Methyl is not a precious darling of chemists. Let's hear what some of them have to say.
SOME EXPERTS WEIGH IN
- "I've never actually used it, but if I did, I'd make sure my affairs were in order first."
-Chemistry employment blogger Chemjobber
- In my 2nd year of grad school, I was working in the lab at night in the winter and there was one other grad student there. All of the sudden he starts opening all the windows in the lab, without explanation. I start closing the windows since it was freaking freezing and I figured he was just being an a##hole, as he was wont to be. So he then starts screaming at me: Do you want to die?' He had spilled a bottle of MM in his hood and was trying to get better ventilation. "
- J.L. Former Wyeth chemist, who prefers to remain anonymous. Can you blame him?
- And finally, an entry from the incomparable Derek Lowe, chemistry blogger to the stars. Not only can the guy write up a storm, but he dabbles in poetry as well. (And the dude gets his own Wiki page too!)
Put In Another Methyl Group: A Villanelle

-Derek Lowe, In the Pipeline, 2010.
I wish I was around when he recited this. Probably wasn't a dry seat in the house.
NOTES:
(1) In the "you just never know what's gonna happen when you wake up on a given day" department, Some nutlog recently read the dimethylmercury article and was arrested by the FBI for trying to buy the stuff online using bitcoin. Just a gentle reminder not to mess with chemists.
(2) Both of these chemicals have caused laboratory accidents which resulted in death. So has Magic Methyl.




