It is difficult to find anything comforting when you are told you have cancer. Perhaps no word evokes more terror, and this is not without reason. In 2022, 1.9 million people in the US were diagnosed with cancer. Even with advances in treatment, such as immunotherapy, the chances of dying from all types of cancer are still disturbingly high. But there is one notable exception: testicular cancer.
Testicular cancer is different
Testicular cancer fundamentally differs from other cancers; the cure rate in men whose cancer has not spread is an astounding 99%. Perhaps more impressive is that even when the cancer has metastasized to the lymph nodes in the abdomen – normally a very bad sign – the five-year survival still ranges from 73-96%, depending on the extent of metastasis. This puts testicular cancer in a class by itself. But why? It can be explained by a little organic chemistry, which will be discussed below.
A Cornell University study explained why the combination of a particular chemotherapy drug (cisplatin, aka cis-diamminedichloroplatinum) and its target, germ cells, is so unique. What is going on? The authors explain: [my emphasis]
Prior to modern genotoxic chemotherapy, the 5-year survival rate for patients diagnosed with metastatic testicular germ cell cancer was only 5%. Remarkably, with conventional chemotherapeutics and surgery, 99% of patients with early-stage disease and 74% of patients with late-stage disease now live at least 5 years...We conclude that the chemosensitivity of TGCTs [testicular germ cell tumors] derives from the sensitivity of their cancer stem cells to DNA-damaging chemotherapy.
Weiss et al., Cell Rep, 2017 Nov 14;21(7):1896-1909. doi: 10.1016/j.celrep.2017.10.078
But there is a downside, too. Cisplatin, although it works miraculously for testicular cancer (1), is one god-awful drug to take. Here's why.
Cisplatin - the ugly side
Chemotherapy drugs are classified by their emetogenic potential - the propensity to cause vomiting (Table 1). Cisplatin does not do well in this regard; it is arguably the most emetogenic chemo drug.

Table 1. Emetogenicity of selected IV chemo drugs. Source: Medscape. The incidence of vomiting (90%) is in the absence of antiemetic drugs such as Zofran, which are quite, but not entirely, effective.
The reason that cisplatin is useful against testicular cancer is the same reason that it is so toxic. The drug is an intercalator (2) – a drug that fits into spaces (called grooves) in the DNA double helix and damages it – the definition of genotoxic.
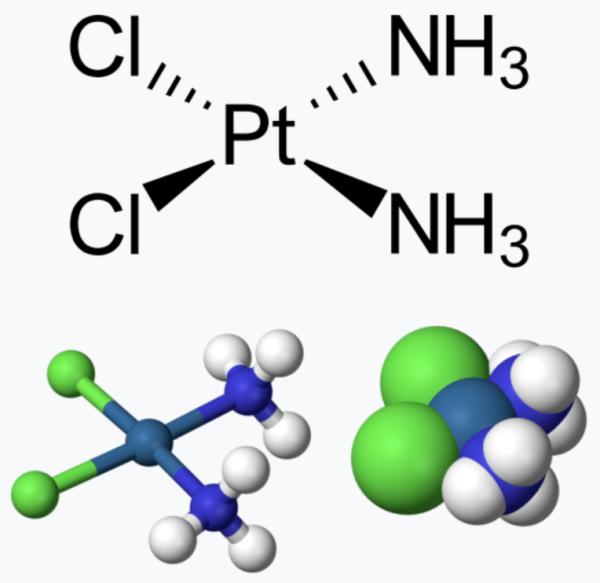
Cisplatin provides a good teaching tool to illustrate the interaction of chemistry, biology, and drugs in modifying disease. In this case, the mechanism of action is the intercalation of DNA by cisplatin. The drug is perfectly "engineered" not only to fit into the grooves of DNA but to stay attached to it, which disables the various DNA repair mechanisms (3). Figure 1 shows the structure of cisplatin and a simplified view of the intercalation process.
Cisplatin's mechanism of action
The simple chemical structure (left) and ball and stick model (middle) both show that the drug is flat (planar), which is unusual for drugs (Figure 1). This planarity gives the drug its particular qualities: the ability to easily insert itself into the spaces that exist in DNA (right). The orange arrows depict the intercalating agent.

Figure 1. (Left) Cisplatin: The green balls are chlorine atoms, and the blue balls are nitrogen atoms. (Right): The black discs represent cisplatin molecules intercalated into DNA. Images: Adapted from 3DChem and Wikipedia.
Because of its size and shape cisplatin looks geometrically like a good intercalator, but you've only seen part of the reason for its effectiveness. The rest can be explained by simple (more or less) organic chemistry. Figure 2 shows a model of cisplatin bound to DNA. The platinum atom (orange circle) remains attached to two nitrogen atoms (blue spheres), but the two chlorine atoms (green in Figure 1) are gone. And the cisplatin is now covalently attached to DNA where the chlorine atoms once were. It is this irreversible attachment that accounts for the extreme genotoxicity of cisplatin.

Figure 2. The attachment of cisplatin to DNA. The pink ovals show the spaces between DNA strands where drug molecules can fit. The orange circle demarcates the platinum atom. Photo adapted from atdbio.
It all comes down to organic chemistry
Let's look a bit closer. Figure 3 shows the reaction at the atomic level. In the figure on the left, we see cisplatin positioned so that the platinum atom is sitting in the correct position and distance to form a bond with the nitrogen atoms of two guanine bases (3). As a result, the two guanine molecules are covalently bound to one platinum. This is called cross-linking - the binding of a molecule to two different sections of DNA. These sections may be on the same strand or another—both damage DNA.

Figure 3: The reaction of cisplatin and DNA (right) The bond that will form is the red hatched line. The product of the reaction (left) now contains two stable platinum-nitrogen bonds.
It is not often that you can look at a drug and its target in such detail and clarity. This is as good an example of the intersection of chemistry, biology, and medicine working together as you'll ever see.
NOTES:
(1) Cisplatin is also used to treat other cancers, for example, ovarian, bladder, head and neck, lung...others.
(2) This is only partly true. Yes, cisplatin is an intercalator, but it packs a double punch. Once the drug enters the DNA groove, it stays there by the formation of a carbon-platinum bond (Figure 3), which keeps the platinum in place.
(3) Some of the reasons that cisplatin is so emetogenic is that gastric mucosal cells, like germ cells, rapidly divide. Damage to mucosal cells also triggers a host of inflammatory processes in the gut.