In the past week, Francis Collins, the former director of the National Institutes of Health, wrote in the Washington Post about his recent diagnosis of an aggressive form of prostate cancer. Like many men, he was initially diagnosed with a slow-growing “grade” of prostate cancer and has pursued a course of active surveillance – a form of watchful- waiting with routine visits to his physician and testing for PSA, prostate-specific antigen, and biopsies. As he writes,
“Over my 40 years as a physician-scientist, I’ve had the privilege of advising many patients facing serious medical diagnoses. I’ve seen them go through the excruciating experience of waiting for the results of a critical blood test, biopsy, or scan that could dramatically affect their future hopes and dreams. … At first, there wasn’t much to worry about — targeted biopsies identified a slow-growing grade of prostate cancer that doesn’t require treatment and can be tracked via regular checkups, referred to as “active surveillance.”
- Francis Collins, MD
Active surveillance or watchful waiting makes logical sense for a slow-growing tumor, waiting to intervene when the tumor shows signs of going rogue and becoming more aggressive. [1] As Dr. Collins writes,
“Early detection really matters, and when combined with active surveillance can identify the risky cancers like mine, and leave the rest alone.”
What is often less frequently mentioned is the anxiety associated with waiting for results.
Ideally, there would be a diagnostic test that would be safe, accurate, non-invasive, and whose results would be both actionable and timely, providing a benefit. A rising PSA on its own lacks enough specificity and requires more invasive testing, like a biopsy, or expensive imaging with MRI, specifically multiparametric magnetic resonance imaging or mpMRI. In these screening scenarios, the negative predictive value (NPV) of a test and its accuracy in detecting disease not requiring further evaluation is most valuable. Studies have placed the NPV of mpMRI at detecting more aggressive GG2 cancers at about 77%, with significant variation for centers and reading radiologists. These tests are not always available based on cost and location.
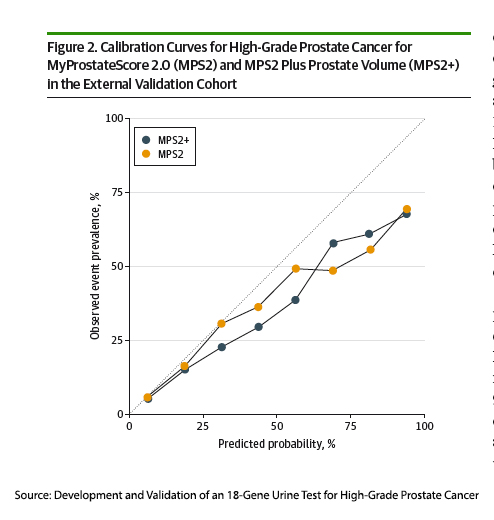
A new non-invasive biomarker, reported in a new study in JAMA Oncology, offers an alternative. There are any number of tests looking at blood or urine for prostate cancer biomarkers. One test of urine, the MPS, marketed as My Prostate Score, used PSA and the expression of two genes associated with prostate cancer to separate the concerning from non-concerning prostate cancers. The new study presents the improved MPS2 version.
The researchers began by searching a large gene panel of patients with prostate cancer for candidate genes associated with high-grade cancers. They identified 54 gene candidates and narrowed that number to a model using 18 genes with and without information on the current prostate size (a very indirect marker of the presence of a tumor). They then obtained urine and biopsy results from a prospective study conducted by the National Cancer Institute’s Early Detection Research Network involving patients undergoing biopsy because of an elevated PSA or abnormal rectal prostate examination. The development group for the new MPS2, 815 patients, had a median age of 63 and PSA of 5.6, which is well above the 95th percentile for this age group, and a 38% incidence of GG2 or greater prostate cancers on biopsy – all real-world numbers.
The validation group involved another 813 patients, median age 62, PSA 5.6, and 20% had GG2 or greater prostate cancers on biopsy. 12.8% were Black, and about a third had previously negative biopsies. The study has limitations, specifically the small percentage of Black patients, given the increased incidence of prostate cancer among Blacks and the population derived from academic centers, both of which make the translation of these results to a more general population imperfect.
Results
 The researchers presented the results based on the area under the curve (AUC). The AUC describes how well a test can tell whether someone has a disease or not. A perfect test would have an AUC of 1, indicating perfect discrimination; a completely random test would have an AUC of 0.5, indicating the discrimination of a coin flip. While useful, it does not provide information about the optimal cutoff point and can be influenced by the prevalence of the condition in the population being tested.
The researchers presented the results based on the area under the curve (AUC). The AUC describes how well a test can tell whether someone has a disease or not. A perfect test would have an AUC of 1, indicating perfect discrimination; a completely random test would have an AUC of 0.5, indicating the discrimination of a coin flip. While useful, it does not provide information about the optimal cutoff point and can be influenced by the prevalence of the condition in the population being tested.
For detecting high-grade cancers, the PSA had an AUC of 0.6, the MPS2 0.82 (0.85 when accounting for prostate size). In other terms, its negative predictive value ranged from 95 to 99%. As a more practical measure, overall, using PSA as the threshold for biopsy would have avoided 11% of unnecessary biopsies, and using MPS2 reduced unnecessary biopsies by 37% (41% when accounting for prostate size).
In the population undergoing initial biopsy, where the rate of high-grade cancers was 26.8%, MPS2+ (the test accounting for prostate size) reduced the need for biopsy by roughly 40%. For those patients undergoing a repeat biopsy where the incidence of high-grade cancer was much lower, 7.3%, MPS2+ eliminated nearly 50% of biopsies. Those are good screening results for any test, let alone one that involves just peeing into a cup.
As the researchers write,
“For individual patients, NPVs approaching 100% provide clear guidance for confident decision-making. For clinicians, uniform use of MPS2 could avoid unnecessary biopsies while preserving immediate detection of 95% of cancers of GG 2 or greater diagnosed using the biopsy all approach. Critically, MPS2 had 99% sensitivity and 99 %NPV for cancers of GG 3 or greater, meaning the rare false-negative.”
[1] Prostate cancers are graded based on their pathologic appearance using the Gleason scale. A Gleason score of 6 or less is considered to be indolent enough to allow for active surveillance. Gleason scores of 7 or more reflect aggressive tumors that require intervention. These tumors are called Grade Group 2.
Source: Development and Validation of an 18-Gene Urine Test for High-Grade Prostate Cancer JAMA Oncology DOI: 10.1001/jamaoncol.2024.045501/jamaoncol.2024.0455




