
A new article in Science Alert describes a serious, rapidly spreading neurological disease in deer, elk, and moose – 800 cases in Wyoming alone. Symptoms include "drooling, lethargy, stumbling, and a vacant gaze."
While this doesn't seem all that different than my average morning it's a potentially serious problem caused by a mysterious pathogen: prions, misfolded proteins that are responsible for mad cow disease in the UK and Creutzfeldt-Jakob disease (CJD) in people (1). The name is a shortcut for "proteinaceous infectious particle" (2)
Although this outbreak is hardly a serious threat to human health at this time it's not impossible that it could become one, especially if prions get into human food, like they did in England in 1996. Between then and 2022, about 180 people died of CJD from infected cows. Apparently, oceans don't stop it; another article reports that two Wyoming hunters who died in 2022 after eating infected venison were the first Americans to succumb to a brain-wasting prion disease. And it's not just Wyoming. Cases have been reported in North Carolina, suggesting a looming spread of the outbreak.
What are misfolded proteins and why are they dangerous?
Almost all pathogens, whether they're as tiny as viruses or as large as amoebas, share a commonality: they carry their genetic information within cells in the form of DNA or RNA. However, prions diverge from this pattern entirely. They lack a cellular structure and any genetic material whatsoever. Instead, they are made up of proteins known as PrPc (prion protein C), typically found in neurons.
For reasons that are poorly understood, prion protein Cs can unfold and then refold into a pathogenic form of the same protein PrPsc (prion protein scrapie) in which the three-dimensional shape of the protein has changed. In other words, the sequence of amino acids – the primary structure – is identical, but the secondary structure – how amino acids interact with other amino acids in the protein – has changed radically. More on this later.
Uh oh. Proteins, amino acids, structures... You know what this means, right? You bet! it's time for yet another...Dreaded Chemistry Lesson From Hell®
And today we have an extra special surprise!

(Left) Steve, our normal co-host of the DCLFR®, left hell for something worse. Hell HR required him to view the entire Barbie Movie, a deeper stage of hell, for purposes of career development. But we are honored to have guest host RFK Jr., step in with some words of wisdom – except for the wisdom part. His co-host Irving (right) appears to be hopelessly confused. Just like Kennedy himself. (Right) Steve appears to be less than enamored with the Barbie Movie, to the point where he attempts self-immolation just to escape. This is generally an ineffective strategy for those who already dwell in hell.
First, a rant!
Before we start, I'm puzzled as to why I'm doing this to myself. For the s### salary I'm getting why should I spend precious days (of the few I have left) putting this miserable lesson together? Our beloved director of medicine, Dr. Chuck Dinnerstein has it figured out. Oh yeah. While I'm slaving away trying to explain complex biochemical processes to a bunch of idiots non-scientists, Chuckie comes up with "I Read a Stop Sign" in the time between flossing and brushing his teeth. Rant over.
Prion disease on the molecular level. It's very cool
Believe it or not, prion disease, much like many other diseases and conditions, can be explained by examining fundamental concepts from Organic Chem 101. It doesn't get much more fundamental than this.
These things are an amides...
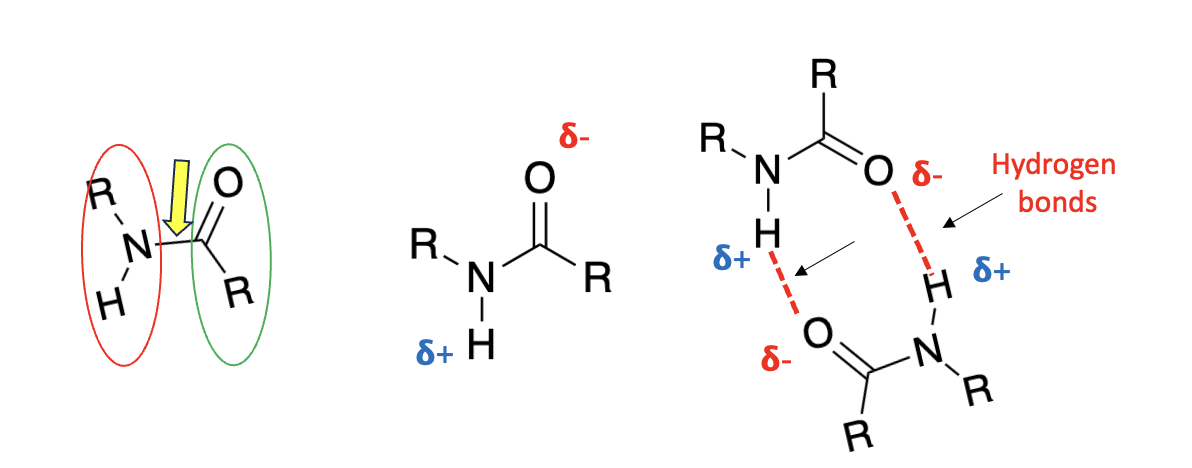
(Left) An amide is the product of an amine (red oval) reacting with a carboxylic acid (green oval). The amide covalent bond (yellow arrow) is quite strong. (Center) The same molecule is drawn to depict an imbalance of charge. Because of the difference in electronegativity between nitrogen and hydrogen, the hydrogen atom has a partial positive charge (delta-plus). Likewise, because of the difference in electronegativity between the oxygen and carbon atoms, oxygen has a partial negative charge (delta-minus). Unsurprisingly, there is an attraction between the two. (Right) Two identical amide molecules are shown interacting via two hydrogen bonds. R stands for any alkyl group.
What does this have to do with prion disease?
Once a normal prion is transformed to its pathogenic form (PrPsc, prion protein scrapie) – the exact process is still a mystery – all hell breaks loose. A cascade results where newly formed pathogenic PrPsc interacts with normal PrPc proteins causing them to misfold – think dominoes – converting them to more PrPsc and this polymerization continues. The most important characteristic of PrPsc is that these proteins stick together, leading to the accumulation of toxic aggregates in the brain. These aggregates kill the cells, causing nerve damage in the brain, which manifests itself as cognitive decline, seizures, dementia, and ultimately death. These conditions are the hallmark of prion diseases.
The chemistry behind the transformation
This is where Organic101 and hydrogen bonding come in handy.
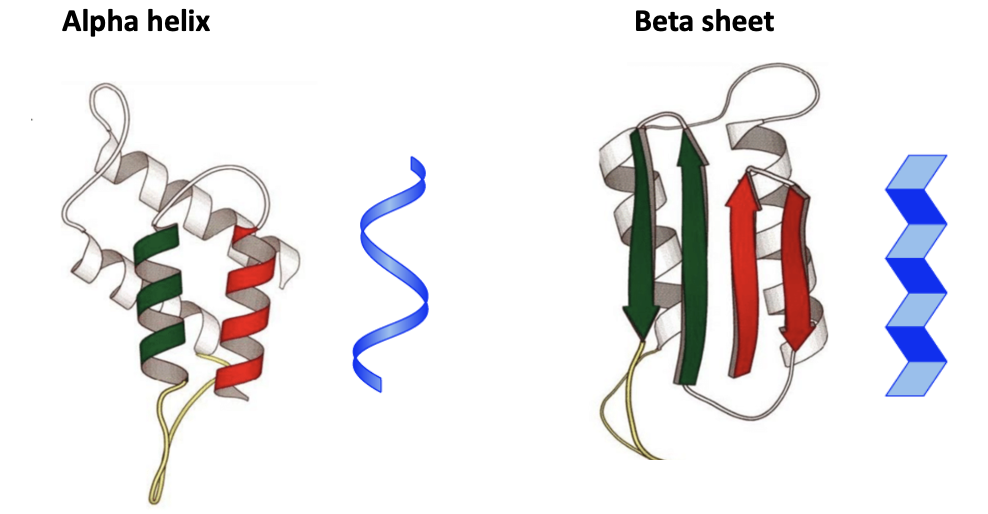
Image credit: Admet
Normal prion proteins exist in an alpha helix (left). The helices are composed of single chains of amino acids – a common 3D structure for proteins, But when PrPc rearranges, the protein takes a different form. The red and green arrows represent four beta-sheets, another common motif in proteins. But this is where the problems arise with prions. In a beta-sheet two single chains are "stuck" together by hydrogen bonding, which is shown on the molecular level below. The blue figures are representations of the shape of both the normal and pathogenic proteins.
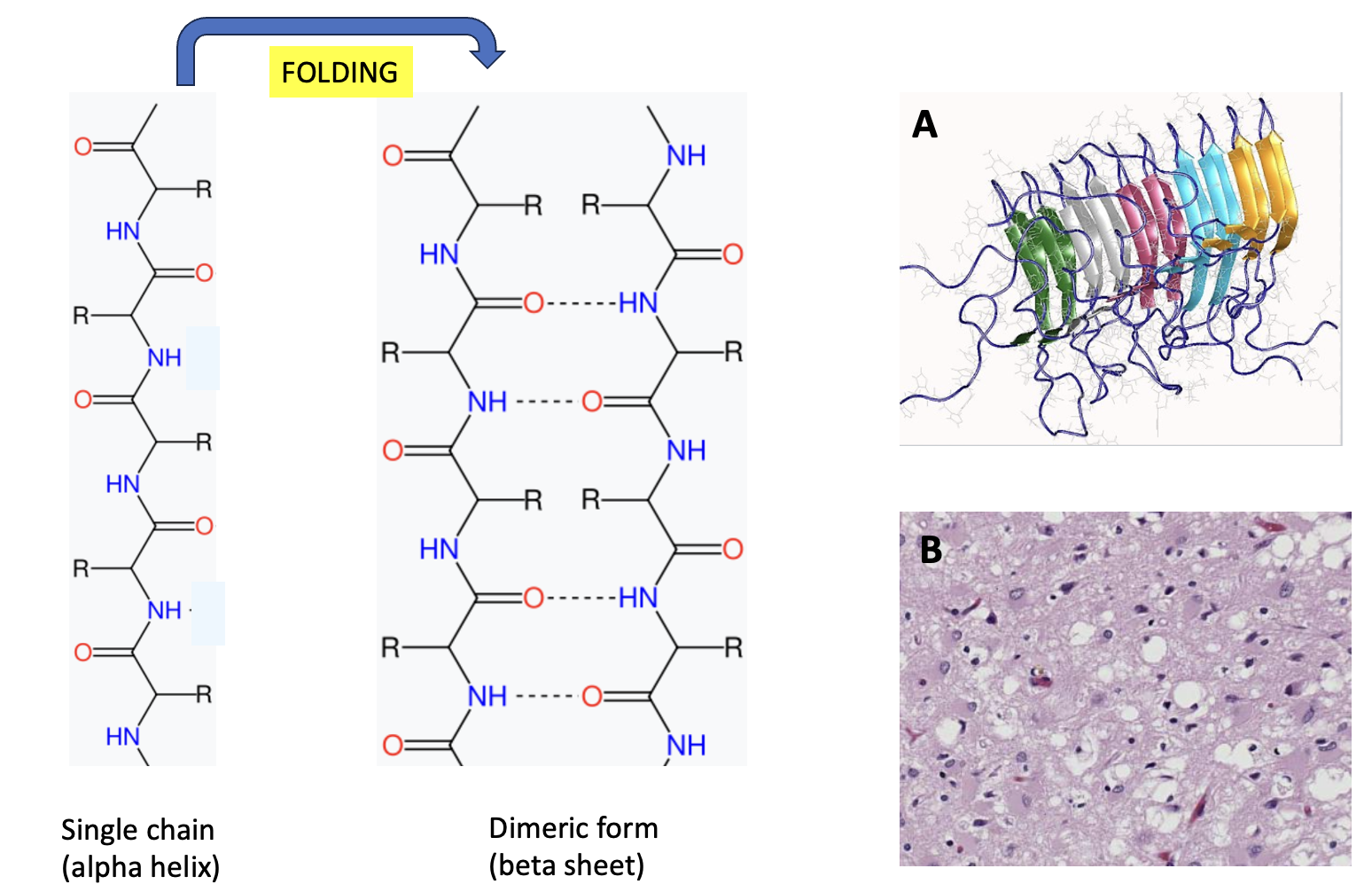
(Left) A segment of a single protein chain in the alpha-helix of normal PrPc. When the protein misfolds two formerly single strands of the proteins are folded together, "stuck" to each other because of internal hydrogen bonding. This results in the formation of four beta-sheets, which change the shape and function of the protein. In particular, the beta-sheets, once formed, result in a cascade where the misfolded proteins form a polymeric clump, which is toxic to brain cells. (Right) (A) An example of five proteins (in this case amyloid) clumped together. Credit: Wikipedia (B) Human brain tissue with Creutzfeldt-Jakob disease has a sponge-like appearance. Credit: Teresa Hammett, Centers for Disease Control and Prevention.
Another way to look at it
For non-chemists, this analogy may help. The unfolded chair represents an alpha-helix. It is impossible to stack them together. But if you take the same chair and fold it, they stack easily. This represents beta-sheets.

Bottom line
With prion disease, the news is mixed. The good news is that (with the possible exception of AD), it is very rare. The bad news is that it is always fatal; People who have Creutzfeldt-Jakob disease typically die within 6-12 months. Nor is there any treatment let alone a cure. Even worse, while normal proteins are degraded/digested in the stomach (just like food) prions are stable in the gut and can be absorbed into the blood and transported to the brain.
Prions are nothing if not interesting. And terrifying.
NOTES:
(1) Other human prion diseases include: Variably protease-sensitive prionopathy (VPSPr), Gerstmann-Sträussler-Scheinker disease (GSS), Kuru, and Fatal insomnia (FI). You don't want any of them. Whether Alzheimer's Disease is a prion disease or simple acts like one is being argued, for example, here.
(2) Are prions pathogen? I would argue yes. They are infectious particles because they can be transmitted from one mammal to another. So I think it's reasonable to consider prion disease to be the product of an infection.


