One of the significant hurdles in the new FDA therapies for Alzheimer’s Disease (AD) is identifying those patients in the early stages of the disease when alterations in the brain have begun, but early dementia may be problematic to diagnose. In making a definitive diagnosis of AD, we require a spinal tap and PET scan – one invasive, the other expensive. Biomarkers in the blood have emerged as increasingly important tools in identifying at-risk individuals who might benefit from early intervention.
The track record in the diagnosis of AD is not good. In a study of patients in memory clinics reported at the Alzheimer’s Association International Conference last week, specialists using clinical examination, cognitive testing, and CT scans identified 73% of patients with AD. Primary care physicians, who often do initial screenings, fared even worse, only 63% accuracy. One of the blood biomarker tests, looking for fragments of amyloid beta and tau proteins, had a diagnostic accuracy of 91%. Another study suggests that
“more than 50% of patients with cognitive impairment remain undiagnosed or incorrectly diagnosed because of the lack of accessible and cost-effective tools.”
Several months ago, the Global CEO Initiative on Alzheimer’s Disease, an AD advocacy group of private and public stakeholders, published a consensus statement in Nature Reviews Neurology on what might be “acceptable performance” for a blood-based screening test for AD.
“blood biomarkers have emerged as scalable tools for clinical evaluation, trial recruitment, and disease monitoring.”
A screening test that is readily performed, inexpensive, and accurate is the key to identifying individuals with early findings of AD. This is especially true when considering populations where the incidence of AD may be low so that screening costs overwhelm the benefits for the few AD cases identified. The graphic describes the parameters considered in the recommendations and are the usual concerns for any screening test.
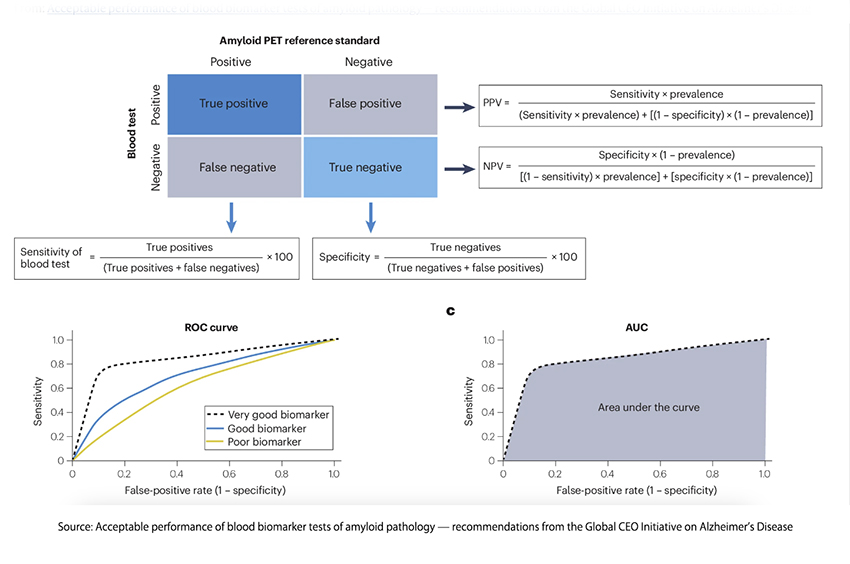
These BBM (Biomarker-based molecular) tests will be used to identify and confirm patients with symptoms suggestive of AD. For screening, BBMs should be used when the belief that AD is present is low – its high negative predictive value serves as confirmation of that belief. For patients where AD is far more likely, BBMs can serve to confirm clinical suspicions. The use of more invasive or costly examinations is thus reduced in both scenarios, making it a practical test in primary care. For those patients identified with AD, BBMs can serve as milestones measuring the progress of the disease or the efficacy of intervention.
The authors of a study of a specific BBM, Phosphorylated tau at threonine 217 (p-tau 217), write:
“With the imminent implementation of anti-Aβ therapies in dementia management, validated blood biomarkers are urgently needed to guide timely treatment decisions.”
Their BBM of choice, p-tau 217, has demonstrated “superior diagnostic accuracy and disease specificity.” It demonstrates increases associated with clinical deterioration, making it, at least in their eyes, “the primary blood biomarker for AD pathology throughout all stages of the disease [AD].”
To reach their conclusions, they used participants in three observational studies, the Translational Biomarkers in Aging and Dementia (TRIAD), Wisconsin Registry for Alzheimer’s Prevention (WRAP), and Sant Paul Initiative on Neurodegeneration (SPIN). None had cognitive impairment at the beginning of the studies, and blood samples were available along with the traditional diagnostic tests involving cerebrospinal fluid (CSF) and PET scanning. They developed a clinical algorithm to determine the best course of diagnostic care.
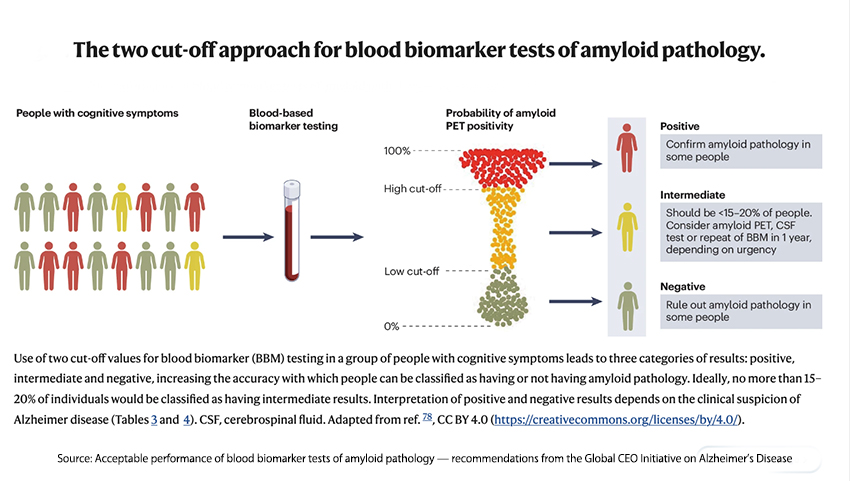
As the Global CEO Initiative recommended, their cutoffs resulted in diagnostic inclusion or exclusion in 80% of patients, leaving only 20% requiring additional testing. That reduction, especially when looking at populations with a low incidence of AD, as seen by primary care physicians, is both medically helpful and a tremendous cost savings.
While cautioning on the generalizability of their study and how translation into the real world of care will reduce the value of these tests, they conclude:
“These results emphasize the important role of plasma p-tau217 as an initial screening tool in the management of cognitive impairment by underlining those who may benefit from anti-amyloid immunotherapies.”
A study reported in medRxiv did a head-to-head comparison of the six commercially available plasma p-tau217 tests and found that they were all equally accurate.
“…regardless of the specific assay, plasma p-tau217 had the highest classification accuracies and correlations with all key outcomes studied—amyloid PET, tau PET, cortical thickness, and cognitive impairment. Thus, it is possible that research studies and clinical trials could rely on a single or limited number of blood measures that are informative regarding multiple aspects of AD.”
The emergence of blood biomarker tests as a frontline tool in the diagnosis of Alzheimer’s Disease (AD) marks a significant step forward in the battle against this debilitating condition. These tests offer a less invasive, cost-effective, and more accurate alternative to traditional methods, potentially transforming how AD is detected and managed, especially in primary care settings. While challenges remain in translating these findings into widespread clinical practice, the promising results suggest that blood tests could soon become vital to early Alzheimer’s detection, ultimately leading to better patient outcomes.
Sources:
Acceptable performance of blood biomarker tests of amyloid pathology — recommendations from the Global CEO Initiative on Alzheimer’s Disease Nature Reviews Neurology DOI: 10.1038/s41582-024-00977-5
Diagnostic Accuracy of a Plasma Phosphorylated Tau 217 Immunoassay for Alzheimer Disease Pathology JAMA Neurology DOI: 10.1001/jamaneurol.2023.5319
Head-to-head comparison of leading blood tests for Alzheimer’s disease pathology medRxiv DOI: 10.1101/2024.06.12.24308839v2
Blood Biomarkers to Detect Alzheimer Disease in Primary Care and Secondary Care JAMA Network Open DOI: 10.1001/jama.2024.13855