Have you ever been to a snooty cocktail party (1) and ordered a Diet Coke from the bar only to get the look? The look – usually withering with a touch of moral superiority – may or may not be followed by the condescending remark. Something like this:
Superior Being: How can you drink that stuff? It's full of chemicals, and it causes cancer.
Me: You're drinking a martini. That's much more likely to give you cancer than any diet soda.
Superior Being: You don't know what you're talking about.
I'm sorry to disabuse Mr. or Ms. Superior Being of their notion, but I kinda do know what I'm talking about, and they probably got their information from Deepak Chopra (who does not). I've written about aspartame (NutraSweet) a number of times. And when it comes to actual cancer risk, Ms. Superior Being is doing something far worse than me and my Diet Coke. That's because drinking alcohol most certainly does cause cancer, while using aspartame does not.
Aspartame
I'm not going to go into the evidence of the risk (lack of risk, really) of the artificial sweetener. Suffice it to say that the FDA, the European Food Safety Authority, and the World Health Organization (WHO) all deemed aspartame safe for consumption. But I will discuss the science behind it.
It's all about metabolism
Chemically, aspartame is the dipeptide formed from two amino acids – phenylalanine and aspartic acid – with a methyl ester cap on the carboxylic acid of the phenylalanine residue (2). When metabolized, it forms these same three components.
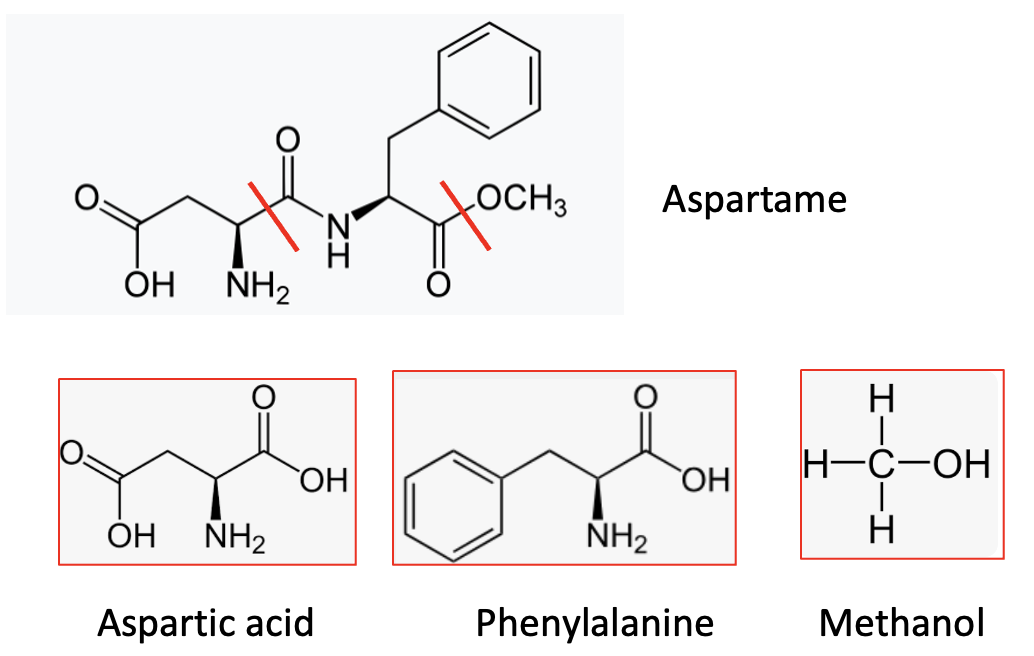
Aspartame (top) is made from (and breaks down to) its two amino acids and one methanol molecule. The red lines indicate the bonds connecting the three components and also where the molecule breaks down.
The primary "knock" on aspartame is due to the formation of methanol when the methyl ester breaks down in the stomach. Here's the reaction:
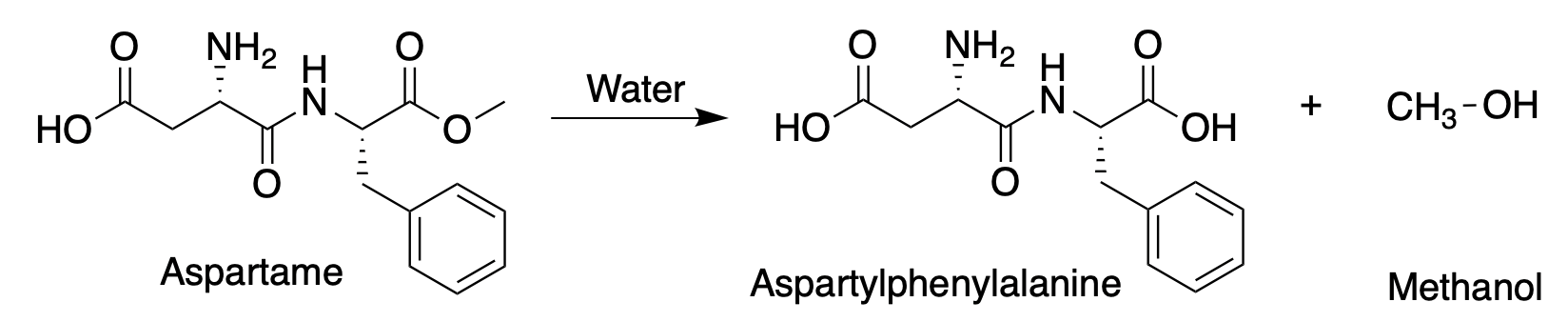
Hydrolysis of the methyl ester in aspartame forms methanol.
Then, that methanol is rapidly metabolized to formaldehyde, a known occupational carcinogen.
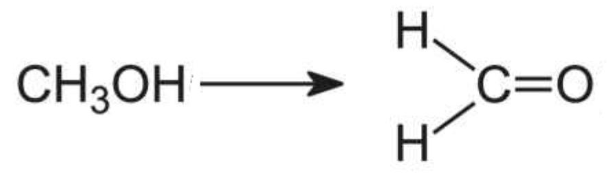
The enzymatic oxidation of methanol forms formaldehyde.
Scary, right?
No. As usual, the dose makes the poison, and in this case, there are other mitigating factors (see below). One Diet Coke contains about 200 mg of aspartame; methanol makes up 10% of the molecule's molecular weight, so one 12-ounce can contains about 20 mg of methanol, roughly the same amount found in a glass of fruit and vegetable juices. Once formed, methanol is rapidly oxidized to formaldehyde, hence the scare. Twenty mg of methanol produces 19 mg of formaldehyde. (3)
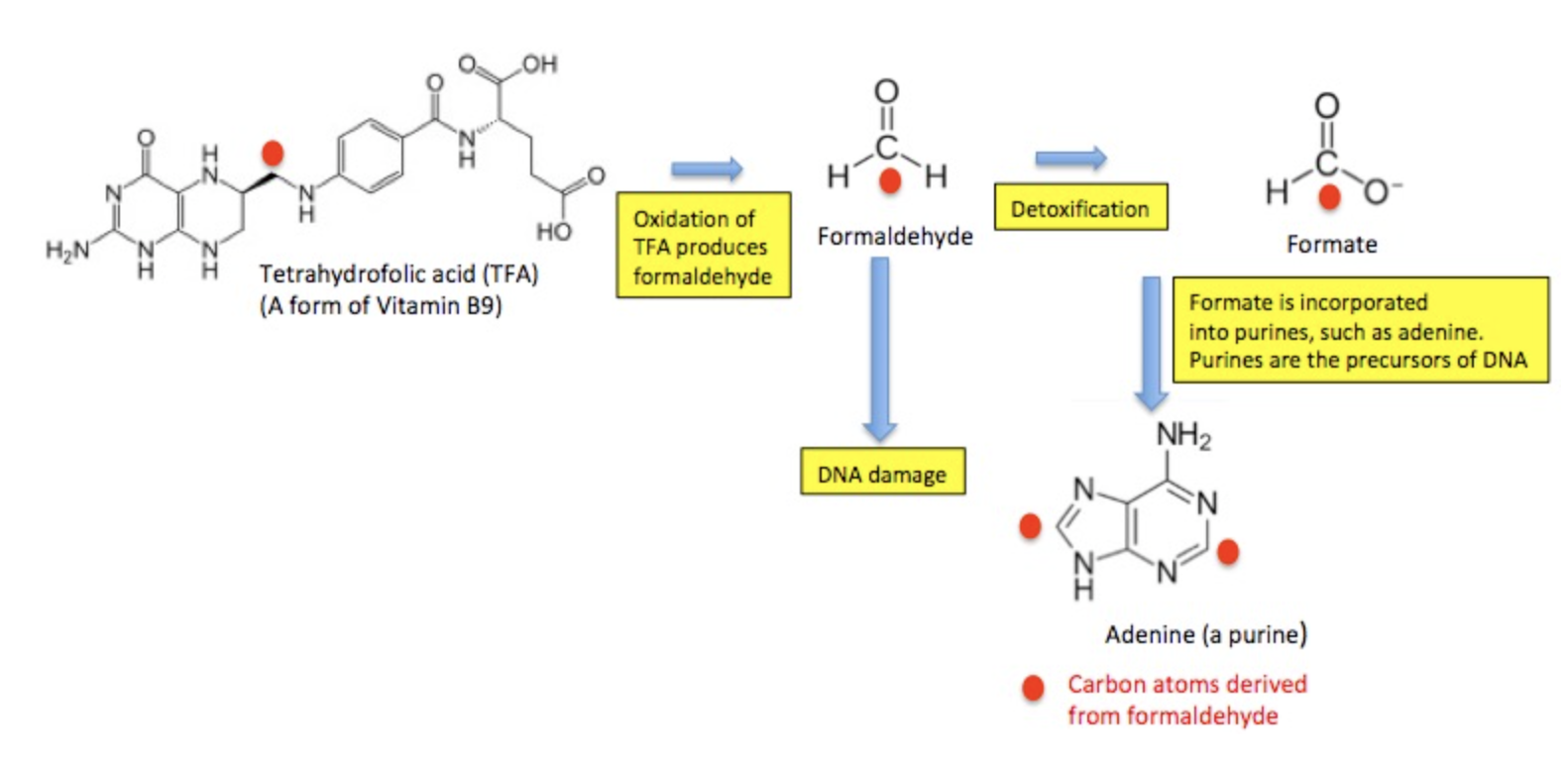
Before you freak out about the formaldehyde in aspartame, keep in mind that even though it is carcinogenic, it is paradoxically essential for human life. Mammals actually make, use, and then destroy formaldehyde. Plenty of it. It has been estimated that we produce 1.5 ounces (about 42 grams) of formaldehyde per day in a biochemical pathway called the one-carbon cycle. (If you're completely out of your mind and want to see how this works, see note 5.) The bottom line is that the daily consumption of 20 mg of formaldehyde is nothing to worry about. Your body is making far more than that endogenously.
Alcohol: A real risk
Compared to the roughly zero cancer deaths per year from diet soda, the consumption of alcohol is far from harmless. According to the National Cancer Institute, alcohol is responsible for 20,000 cancer deaths (4). And, according to the World Health Organization (WHO), alcohol is responsible for around 4 percent of all cancer deaths globally. This translates to hundreds of thousands of deaths each year worldwide. Why all the cancer? If you need to point fingers, point them at acetaldehyde, the first metabolite of ethanol.
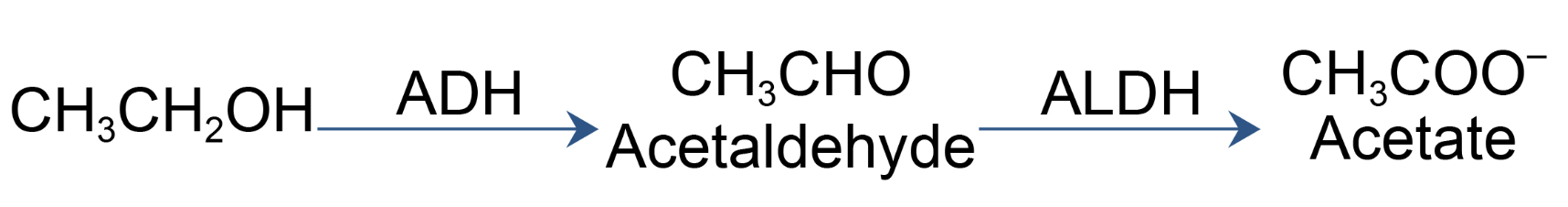
The metabolism of ethanol is a two-step process beginning with the oxidation of ethanol to acetaldehyde, which is promoted by the enzyme alcohol dehydroxylase (ADH). Let's do some math. A can of beer or a glass of wine contains 14 grams of alcohol. Since virtually all alcohol is metabolized via conversion to acetaldehyde, we can estimate that even a single drink will expose you to 14 grams of acetaldehyde – 700 times more than you'll ingest from the formaldehyde you get from one can of Diet Coke.
Acetaldehyde is also carcinogenic. This is a real problem
Formaldehyde and acetaldehyde are carcinogenic because they both react with the nitrogen atoms of DNA bases and can form cross-links between two DNA bases within the same strand (intrastrand cross-links) or between different strands (interstrand cross-links). Both of these processes damage DNA (genotoxic), leading to cellular mutations.
Of the two chemical toxins, formaldehyde is a more reactive electrophile and reacts with DNA about 10-100 times faster than acetaldehyde. It seems we're trying to balance the risk of a very small amount of a very reactive chemical (formaldehyde) with a much more significant amount of acetaldehyde, which is less reactive, but still significantly so. Who "wins?"
It's not that simple
Although formaldehyde is inherently more chemically reactive and more likely to react with DNA, a whole lot more acetaldehyde is formed from drinking alcohol than formaldehyde when consuming a Diet Coke.
But, as I mentioned before, the body has evolved a way to make its own formaldehyde for use in the one-carbon cycle, which enables the body to synthesize nucleic acids (for DNA and RNA biosynthesis), amino acids, and vitamins B6, B9, and B12. Then, excess formaldehyde is destroyed by an enzyme called formaldehyde dehydrogenase (FDH), which uses glutathione as a cofactor. There is no corresponding mechanism in mammals for acetaldehyde, so it persists longer than formaldehyde.
Bottom line
Both formaldehyde and acetaldehyde are recognized as carcinogens. Although acetaldehyde may be somewhat less so, people who drink alcohol are exposed to quite a bit of it (6). People who use aspartame will be exposed to minuscule amounts of methanol (hence formaldehyde) which will rapidly be detoxified.
If you're looking to cut your cancer risk, you'll be far better off having a Diet Coke instead of wine, beer, or hard liquor.
NOTES:
(1) If you live in Manhattan, the answer is almost surely yes.
(2) Residue is a term used in chemistry to describe any of the 20 amino acids that make up proteins.
(3) This is because their molecular weights are almost the same. Methanol is 32, and formaldehyde is 30.
(4) This number reflects only alcohol-related cancer deaths. The total number of alcohol-related deaths in the US is about 180,000 annually.
(5) Knock yourself out:
(5) Kuykendall JR, Bogdanffy MS (1992). "Efficiency of DNA-histone cross-linking induced by saturated and unsaturated aldehydes in vitro." Mutat Res. This paper provides comparative insights into the efficiency of aldehyde-induced DNA-protein cross-linking, highlighting formaldehyde's higher reactivity.
(6) Some people lack the enzyme that would normally detoxify acetaldehyde, converting it into acetic acid. Since it is commonly found in Asian people, the effects – facial flushing, tachycardia, nausea, and headaches are sometimes referred to as the “Asian flush syndrome," which comes from the buildup of acetaldehyde in the blood.
#Disclaimer: Neither ACSH nor I get any compensation or funding from the Coca-Cola Company. I drink Diet-Coke for the same reason I always have. I like it. And Diet-Pepsi tastes like moose urine.




