
The other day on Fox News’ Jesse Waters Primetime, Calley Means, co-founder of TruMed, a B2B company [1], spoke on how to get the corruption out of scientific guidelines and get at the root causes of why people become ill. He asked a few questions that I thought I might answer.
“Let's get the corruption out of the scientific guidelines. Why are 95% of the FDA advisors taking money from food companies? … Why is 75% of the FDA drug approval program funded by Pharma? Why are there still no federal nutritional guidelines for school food subsidies?
Why is 75% of the FDA drug approval program funded by Pharma?
The short answer is that it is required by laws put in place by the US Congress. From its inception, the FDA’s drug approval process was fully funded by US taxpayers until 1981, when the first case of AIDS was reported. For those with a short or no memory, AIDS was a medical crisis certainly commensurate with our more recent medical and cultural experience of COVID. Unlike COVID, where a vaccine was rapidly developed via Operation Warp Speed, finding a treatment for AIDS took far, far longer. AZT, the first effective treatment, was approved in 1987. But activists, in the words of the FDA, “were incensed about long delays in getting experimental HIV drugs studied and approved by the FDA.”
In 1992, the FDA was understaffed and had a slow, unpredictable process for premarket drug review and a backlog of drugs awaiting approval. Congress passed the Prescription Drug User Fee Act (PDUFA), signed by President Bush, charging manufacturers fees when applying for a drug review – which has since been expanded to medical devices, OTC drugs, and biosimilars. This user fees are paid in advance, irrespective of the outcome of those reviews.
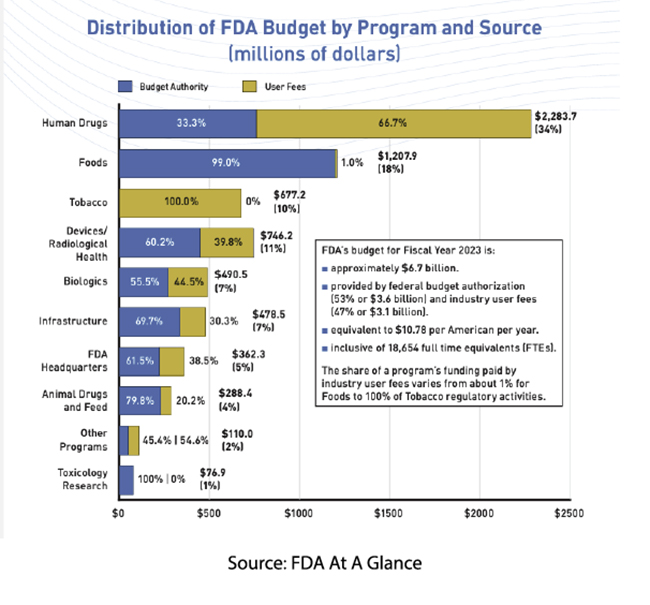
Currently, 65% of the funding for human drug regulatory activity comes from manufacturers, which is slightly less than the FDA’s overall budget. (We will not quibble over Mr. Means's slight exaggeration.) In exchange for those fees, which this year run slightly over $4 million for reviewing a new drug requiring clinical data, manufacturers have negotiated performance measures to speed up FDA review and make the review process more predictable – a valuable quality in business. The monies go primarily for the salaries of the staff needed to carry out activities that Congress has specified that user fees cover, e.g., monitoring of research, review of human drug applications, and post-market safety activities.
As reported in The Conversation, user fees have had mixed impacts
- The new drug approval process has gone from 29 months to 10 months
- First-time approvals have gone from 38% to 61%
- Post-market black box warnings, the highest level of safety alert or drug removal from the market, have gone from 21% to 27%
You can judge the value for yourself. In either case, we, the people, will pay the piper for regulation – you can spread the cost out to the taxpayers or the pill-takers, but in the end, we pay the bills.
Why are 95% of the FDA advisors taking money from food companies? … Why are there still no federal nutritional guidelines for school food subsidies?
These two questions revolve around the current updating of the school's nutritional standards. Mr. Means is incorrect in stating that there are no federal nutritional guidelines; they have been published, and their implementation will be phased in over the next few years.
Alternatively, Mr. Means is correct; 19 out of 20, 95%, of the members of the advisory committee to the USDA on school nutritional standards take money from food companies. However, I hasten to mention an old quote from Willie Sutton, a 1930s bank robber, who was asked why he robbed banks. He responded that that was where the money was. In a similar sense, when seeking expertise in nutrition and many other areas, the money that has helped create their knowledge comes from a range of deep-pocketed advocacy groups. [2]
The 2025 Dietary Guidelines Advisory Committee was formed under the rules of the Federal Advisory Committee Act (FACA), requiring:
- “At least ten years of experience as an academic, researcher, practitioner, or other health professional in a field related to one or more of the scientific topic areas to be examined…
- Advanced degree in nutrition or health-related field…
- Expertise related to one or more of the scientific topic areas to be examined by the Committee as demonstrated by the number and quality of peer-reviewed publications and presentations
- A Committee that is reasonably balanced in terms of points of view and expertise, experience, education, and institutional affiliation, with a goal of establishing a diverse membership that is reflective of the racial, ethnic, gender, and geographic diversity within the United States.”
That third requirement, mandated by our representatives in Congress, results in individuals funded by advocacy groups with strong nutritional concerns. Mr. Means and his co-founder Justin Mares's concerns are for financial conflict of interest and groupthink.
We can dispense with the financial conflicts by pointing out that all of these appointed individuals are special government employees (SGE) and are subject to 18 USC § 208, which
“prohibits all employees, including SGEs, from participating personally and substantially in any particular matter that has a direct and predictable effect on their own financial interests or the financial interests of others with whom they have certain relationships. In addition to an employee’s own personal financial interests, the financial interests of the following persons or organizations are also disqualifying: spouse; minor child; general partner; organization which the individual serves as officer, director, trustee, general partner or employee; person or organization with which the employee is negotiating or has any arrangement concerning prospective employment.”
I believe there is a stronger argument to be made around groupthink and that, in general, a more diverse scientific representation is essential, especially in areas where the consensus is ill-formed or uncertain, as is the case concerning nutritional requirements. Gathering a range of opinions requires expertise in the field and an openness to other points of view – which is difficult in most settings. I would also argue that when it comes to implementing these guidelines, the advisory committee is woefully shy of the individuals with application expertise, those involved in the logistics of supplying food to schools and preparing those meals.
The problem I have with Mr. Means is that there is a kernel of truth in what he says. We would be better served by more diverse expertise, but that doesn’t mean Robert F. Kennedy, Jr should have a seat at the table; he fails the first three Congressionally mandated criteria for serving as an advisor. I have a greater problem with what Mr. Means fails to say, for example, that Big Pharma’s FDA support was a decision made by representatives of we, the people, or that in nearly every instance, those with expertise have had their training and experience funded by advocacy groups – that is, after all, had capitalism works.
In conclusion, the concerns raised by Calley Means about conflicts of interest within the FDA and public health guidelines echo a larger, ongoing debate about the influence of corporate funding on regulatory bodies. While Means and others, like Robert F. Kennedy Jr., highlight legitimate issues about transparency and accountability, it’s essential to recognize that many of these structures are deeply embedded in the legal and financial frameworks established by Congress. Reforming these systems to ensure more diverse, independent perspectives will require more than rhetoric—it demands thoughtful policy changes that balance expertise, funding, and public trust in the health and safety of all Americans.
[1] TruMed is a company that facilitates the expenditure of employee Health Saving Account (HSA) money on their client’s health-related products. “Truemed connects customers with a medical practitioner to determine what products or services may treat, mitigate, or prevent certain health conditions….With a Letter of Medical Necessity, specific purchases can be paid for with or reimbursed from HSA/FSA money, saving an average of 33%!” Among the product categories are fitness, sauna and cold plunge, supplements, and health tech.
[2] Among the funders, you will find any number of “Bigs,” Egg, Beef, Candy, Diabetes, Philanthropy, Pharma, Poverty, Breastfeeding, and Academia



