While the amount of plastic waste floating around our oceans has been debated, whatever the real number is, it's very large. So it is not surprising that there is a fair amount of research into ways to degrade different types of plastics (See Some Hope For Plastics In The Environment?).
Although some clever science is being used to address the problem there is no practical method that is capable of handling the enormous amount of plastic that has been tossed away. But chemists at Swansea University in Wales and Cambridge University have come up with a method that uses sunlight to not only break down plastics but also generate hydrogen, the worlds cleanest fuel, in the process. Although this experiment is only at the proof-of-concept stage, it has the potential to be more.
‘This process demonstrated its great environmental and economic value in the real world, given its significant potential in converting the huge amount of plastic wastes to produce valuable chemicals and fuels."
Shaohua Shen, (a researcher in nanomaterial-based solar hydrogen generation), Jiaotong University, China.
Taylor Uekert and colleagues writing in Energy & Environmental Science (1) described a method called photoreforming that involves water, sodium hydroxide, sunlight, and a cadmium-based catalyst to break down plastic. Although the catalyst, which is called a CdS/CdOx quantum dot (QD) photocatalyst (2), is unlikely to be mentioned at your next cocktail party, the authors describe it as "inexpensive." And the procedure for using it is simple; it is done at a normal temperature and atmospheric pressure - a big advantage.
Here's how it works:
- The catalyst is put onto the plastic.
- Then the plastic is put into a "test tube" containing a concentrated solution of sodium hydroxide (lye).
- The tube is put in the sun.
- The plastic decomposes into small organic molecules and hydrogen is generated.
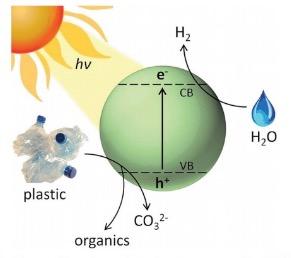
A diagram from the paper (Figure 1) tells us what is going on at the atomic level. Sunlight strikes the cadmium sulfide which causes the ejection of a high energy electron. The electron reacts with water to produce hydrogen gas and also degrades the plastic to produce small fragments (depending on the plastic) of benign organic chemicals such as lactic acid, acetic acid, and alcohol.
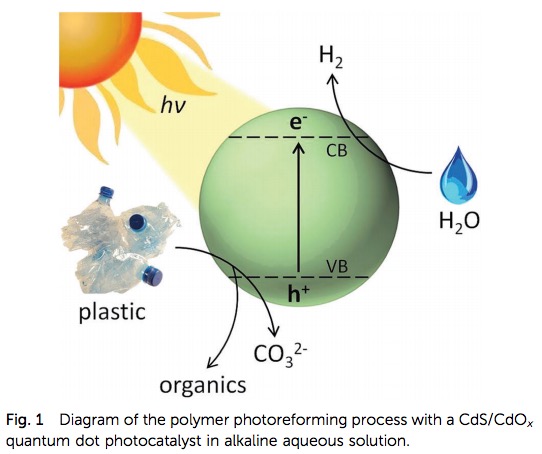
Figure 1. A schematic diagram of the photoreforming process. Source: Energy Environ. Sci.
Another advantage of this method is that other methods for degrading recycled plastic are sensitive to food residues that are inevitably found in plastic waste because these can "gunk up" the catalyst and stop the reaction. But the photoreforming method isn't picky about what it "eats" - plastic is fine and so is the stuff that sticks to it.
How might this process be used? One of the co-authors Moritz F. Kuehnel says:
‘Our vision is that this will be an additional way of treating non-recyclable waste. We could scale up the process and use it for treating the leftover waste in a recycling plant. Ultimately, maybe people could treat their own plastic waste in their gardens, similarly to compost, with a solar waste-reforming device. You put your plastic waste in it and get hydrogen to heat your house or fuel your car.’
Moritz F. Kuehnel, Ph.D.
There is a very long road from dissolving scraps of plastic in a lab to running your car with the hydrogen that is generated, but this procedure requires no special equipment, only readily available materials and light. Very clever.
NOTES:
(1) Original reference: T Uekert et al, Energy Environ. Sci., 2018, DOI: 10.1039/c8ee01408f
(2) CdS is cadmium sulfide, (CdO)x represents a mixture of cadmium oxide and cadmium hydroxide, which covers the cadmium sulfide and protects it from being destroyed by the light.