Let’s get right to it. If you need a catch-up on the aducanumab controversy, you can look here and here for the FDA perspective. The study was simple. The researchers used available CMS beneficiary data from 2018 to determine how many might be eligible, based upon documented diagnosis, for aducanumab – those with mild cognitive impairment (MCI), those with Alzheimer Disease (AD), and those with Alzheimer Disease and related disorders (ADRD). [1].
- 27,785,076 Medicare beneficiaries yielded
- 2,870,023 with ADRD (10.3%)
- 1,026,387 with AD (3.7%)
- 396,251 with MCI (1.4%)
- The potential beneficiaries of aducanumab, based solely on diagnosis, over 4 million or 15.4% of individuals enrolled in the Medicare program. A very big market.
The results of the studies that the FDA used in making their determination are controversial; we can argue that back and forth. But we cannot argue that the participants were carefully selected; there were exclusions based upon age (over 85), chronic renal disease, the use of anticoagulants, and underlying cardiovascular disease. The researchers looked at each beneficiary for those specific exclusions – would these individuals be eligible to be in the studies?
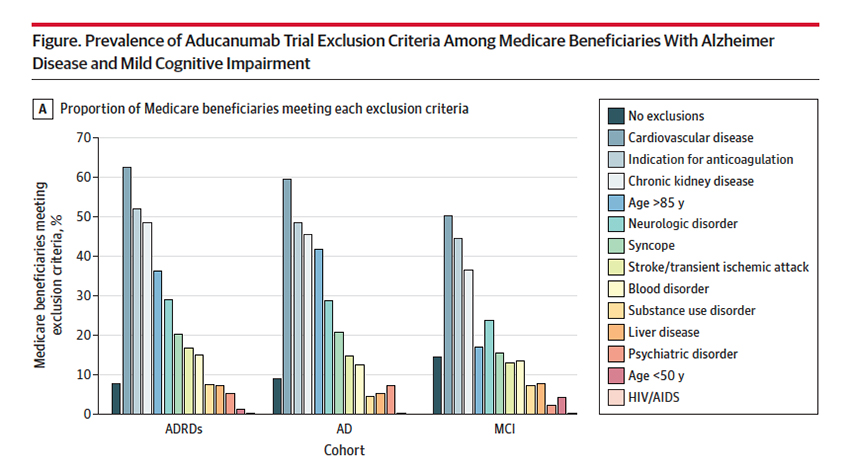
85.5% of those with MCI, 91% with AD, and 92.2% with ADRD had exclusion criteria making them ineligible for the study. More importantly, given the equivocal findings of the study, there is a solid case for excluding them from receiving aducanumab; after all, the study exclusions were designed to pick the patients at least risk and with the greatest possible benefit. In many instances, eligible Medicare beneficiaries had met more than one exclusion criteria.
The FDA has, subsequent to their original determination, narrowed the indication to those with MCI, who have the most to gain and the least to lose. But that leaves 85% at risk. As the authors suggest, and I would agree, the FDA should restrict prescription to those beneficiaries that would not have been excluded by the study’s exclusion criteria. That would serve as a reminder to physicians who can prescribe the medication off-label. It would also help to limit its use to cases where it may show value. Aducanumab’s approval means that the additional post-marketing research to be done by Biogen is done on our dime – we are paying for the medication, Biogen collects the data. If we are spending the money to do further research, don’t you think the FDA should at least continue the contraindications the controversial studies had in place?
[1] The seeming redundancy in the diagnosis is a product of our coding schemes
Source: Representativeness of Participants Eligible to Be Enrolled in Clinical Trials of Aducanumab for Alzheimer Disease Compared With Medicare Beneficiaries With Alzheimer Disease and Mild Cognitive Impairment JAMA DOI: 10.1001/jama.2021.15286




