The term "smoking gun" doesn't even begin to describe this one – an alleged murder by poisoning that happened in Minnesota.
The facts according to NBC
- A man's wife was poisoned. Who's the prime suspect? The husband, of course. But it gets worse.
- The husband was an expert in chemical poisoning. (This will not help the defense.)
- The poison used was colchicine, a rather toxic gout drug.
- The husband, Connor Bowman, a former resident at the Mayo Clinic in Minnesota, did online research on colchicine, one of 19,000 FDA-approved drugs. (Neither does this.)
- The victim, Betty Bowman, also worked at Mayo. She did not have gout, yet colchicine was found in her body. (Or this.)
- After Ms. Bowman's death, Connor Bowman tried to stop an autopsy and instead have her immediately cremated, claiming that she had a rare disease.
- He was arrested with a deposit slip for $450,000 of the $500,0000 life insurance policy he bought.
- By even the most forgiving of standards, things do not look good for Connor.
Of course, all of these facts are based on media reports, which are not habitually reliable on a good day. Still,
I would not want to be the defense attorney stuck with this one. But, no matter how bad the facts are there's always hope.

Photo: Charles LeBlanc, Flickr
What is colchicine?
Colchicine is an alkaloid (1) found in the autumn crocus (Colchicum autumnale) and several other plants); it was developed by the plant(s) as a defense against being eaten by herbivores and insects. Its toxicity makes colchicine an effective deterrent. But despite also being toxic to humans, colchicine is an FDA-approved drug primarily indicated for gout (2). Because of its toxicity, colchicine suffers from a problem that is common to a number of drugs – a low therapeutic index, aka a narrow therapeutic window. More on this later. (You should also read Chuck Dinerstein's recent article about how colchicine may be useful in treating heart disease.)
Throughout history, chemicals (or their synthetic derivatives) from plant extracts have led to the discovery of many important drugs. Colchicine is one of these; for gout, it works by decreasing inflammation. (The most widely used gout drug is allopurinol, a 75-year-old drug, which operates by an entirely different mechanism – the inhibition of uric acid production.) (3)
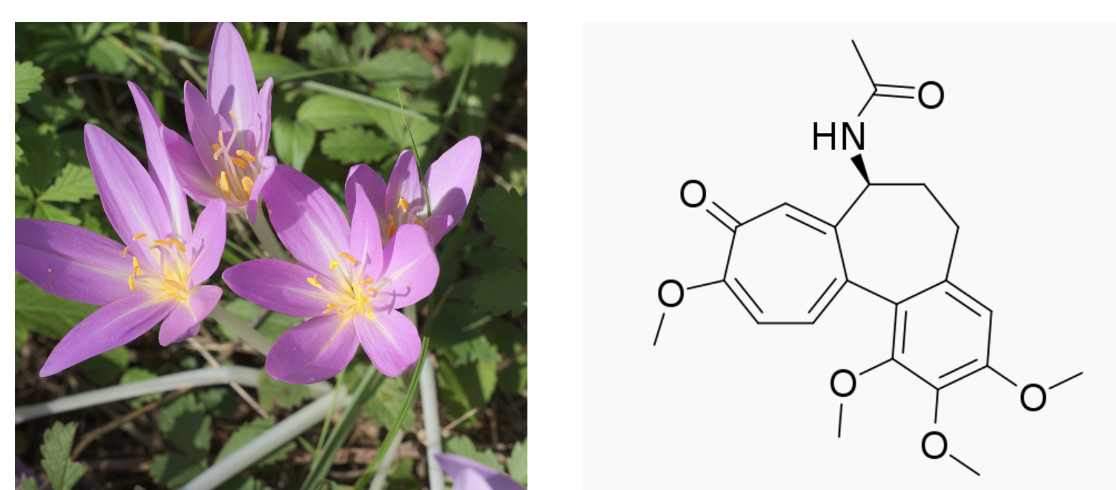
(Left) Autumn Crocus. Beautiful and potentially deadly. Photo: Wikimedia Commons. (Right) The chemical structure of colchicine. Looks like a spaceship, no?
With colchicine, the dose really does make the poison
The maximum daily dose of colchicine is 1.8-2.5 mg per day; a lethal dose can be as little as 3 mg (4), but 7-25 mg is more commonly cited as the lethal dose. Still, the problem is obvious; the maximum therapeutic dose of the drug is disturbingly close to the minimum lethal dose, making an overdose a real possibility. This is not something most of us would not willingly volunteer for since colchicine poisoning symptoms include:
- abdominal pain
- nausea, vomiting, and diarrhea
- severe dehydration
- bone marrow failure
- multi-organ failure
- mental status change
- heart failure
- shock
Some other drugs with a narrow therapeutic window include warfarin, lithium, digoxin, and phenobarbital. Tylenol too.
Why is colchicine toxic?
Colchicine is a microtubule inhibitor – a drug that prevents the formation of the mitotic spindle, which is an essential process in dividing cells. It ensures the correct placement of new chromosomes in daughter cells.
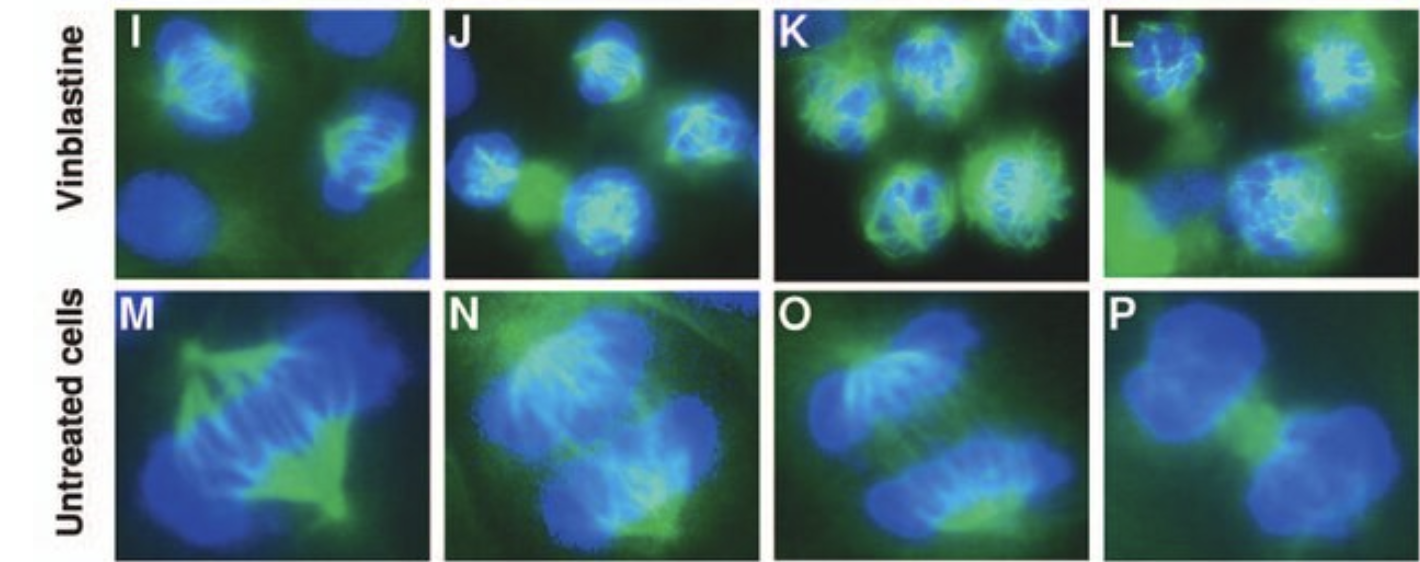
The mitotic spindle in the presence (at different concentrations) and absence of vinblastine (very similar to vincristine). Source: "In vitro and in vivo anticancer activities of synthetic (-)-laulimalide, a marine natural product microtubule stabilizing agent." Anticancer Research 27(3B):1509-18, May 2007.
Those of you who are familiar with cancer chemotherapy may recognize these terms. The chemo drugs vincristine and vinblastine, vinca alkaloids, although they come from a different plant, Catharanthus roseus, aka the Madagascan Periwinkle, work in the same manner – inhibiting cell division. (It is not a coincidence that colchicine is also a potential cancer drug.) The toxicity is why you see vinca commonly planted in areas where deer eat other flowers. The plant is toxic. (5)
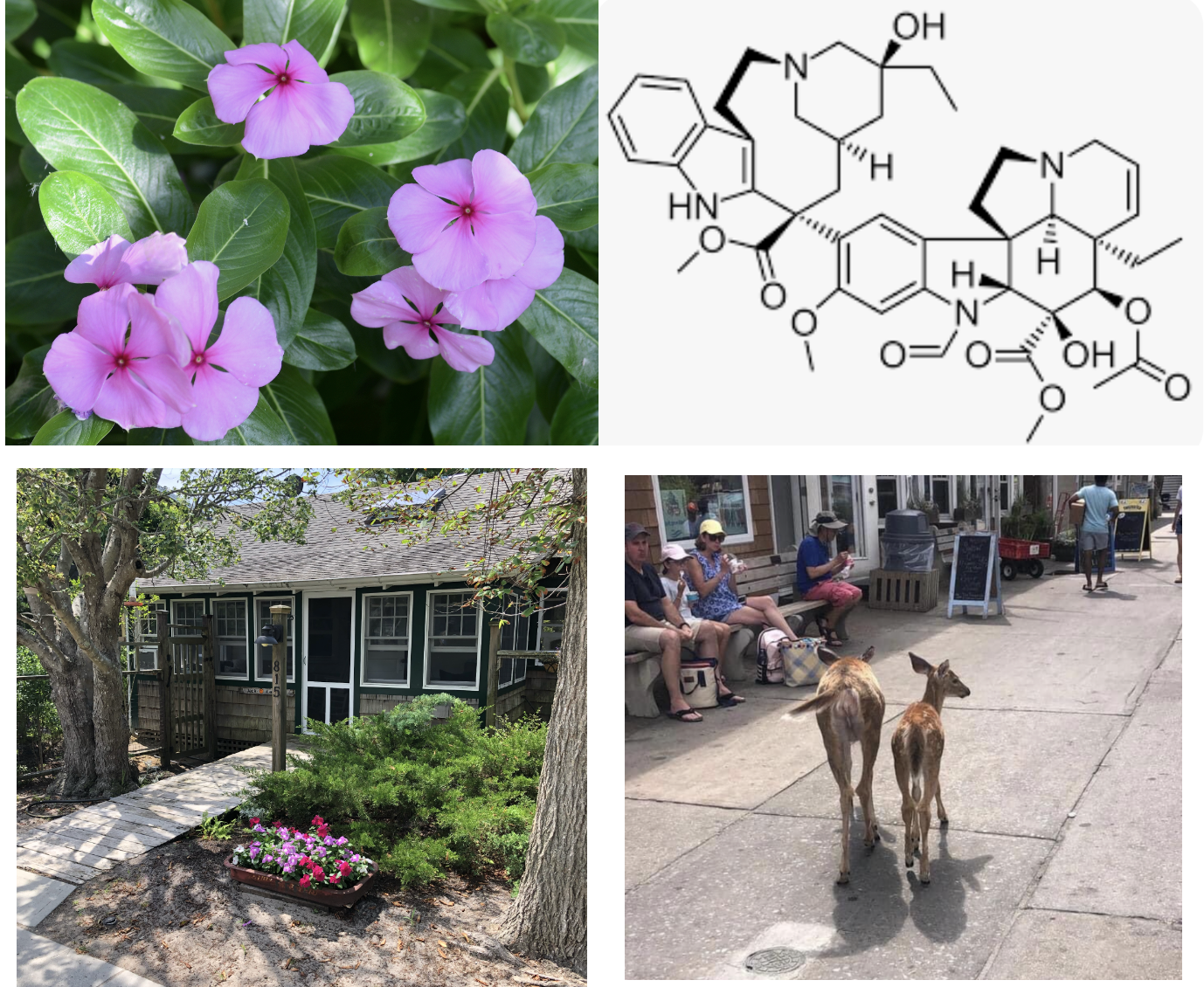
(Left top) Catharanthus roseus, aka the Madagascan Periwinkle, a member of the Vinca plant family, is the source of the cancer drug vincristine (Right top) Nice structure, huh? (Bottom) This is why I plant vinca in front of my house, a place where we have a bit of a deer issue.
Update 10/30/23: It's quite a coincidence, but in medical news today, one day after I wrote this, there is a big story about how scientists have been wrong for decades about the details of how this process works. And I'm one of them. My Ph.D. was based on semisynthetic analogs of vincristine as potential cancer drugs. Weird? Oh, yes. And it's Halloween.
NOTES:
(1) An alkaloid is a nitrogen-containing natural product found in plants. Of course, organic chemistry is never straightforward. Usually (but not necessarily), an alkaloid contains a basic nitrogen. Dweebs chemists who look at the structure of colchicine will immediately see that there is a nitrogen atom, but it is not basic. Does this make it an alkaloid? Most would say yes, although the term "protoalkaloid" has been coined for situations like this.
(2) Colchicine is also used to manage familial Mediterranean fever (FMF) as well as certain other inflammatory conditions.
(3) Uric acid, a water-insoluble waste product formed from purines (which can arise from a high meat diet), forms crystals, some of which are sharp, in joints. This is why gout is so painful. Allopurinol is an inhibitor of the enzyme xanthine oxidase, the enzyme responsible for converting hypoxanthine to uric acid.
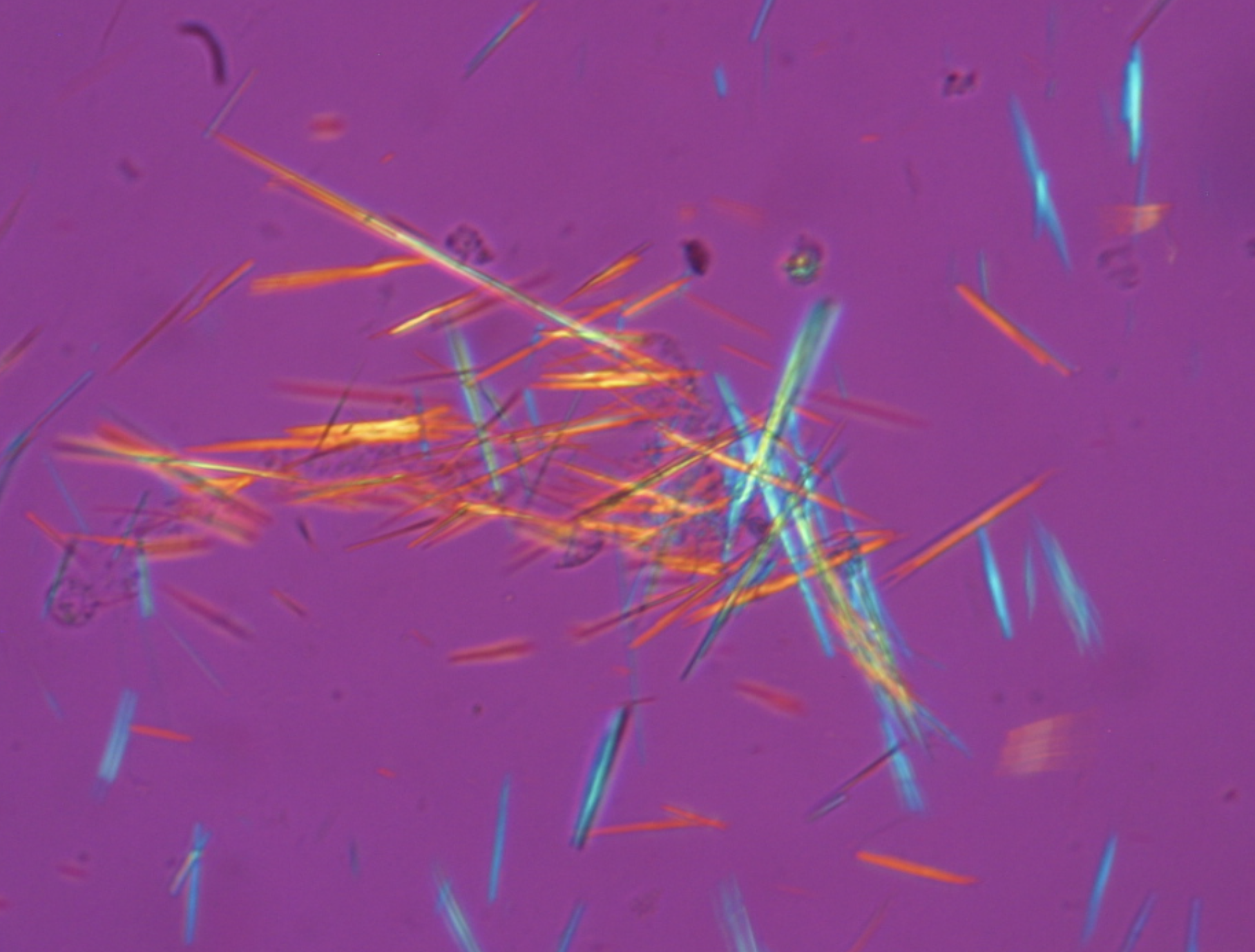
Uric acid crystals from synovial fluid visualized under polarized light. Ouch. Image Wikimedia Commons
(4) This low dose was reported in a single case study from 1934.
(5) This doesn't always work. It is not uncommon to see the flowers nibbled, especially early on. The deer bite off a flower, then decide it's not edible and drop it. Then the damn things bite off the next flower as if to say, "Maybe this one will be OK," and drop that one. This may continue until all the flowers are gone. Duh.




