Disclaimer: For those of you who read this article for purely prurient reasons I must regretfully inform you that there is a real chance you may actually learn something. If this is unacceptable and you don't want to learn anything – at least anything factually accurate – perhaps you might try Dr. Axe's website, which Katie Suleta thoroughly eviscerated in a recent article.
One of the maniacs I work with sent me a paper titled "Why urine is yellow" article as if I'm the "Kidney King" or "Wizard of Whiz." (The actual title of the article is a bit more obscure: "[Bilirubin reductase] is a gut microbial enzyme that reduces bilirubin to urobilinogen.") This has little appeal. But I'm always reluctant to pass up a good chemistry lesson, especially one that might be humorous, if not downright idiotic. Perhaps this is both.
Why is urine yellow?
First of all, this is not necessarily correct. True to form, I've written about pee colors before. If you want to piss away even more of your time, see Pink, Orange, And Green Urine & A Color Chemistry Lesson? Oui (2020) and also Why Andy Warhol Peed On His Paintings (And Worse) - A Perverted Chemistry Lesson (2017).
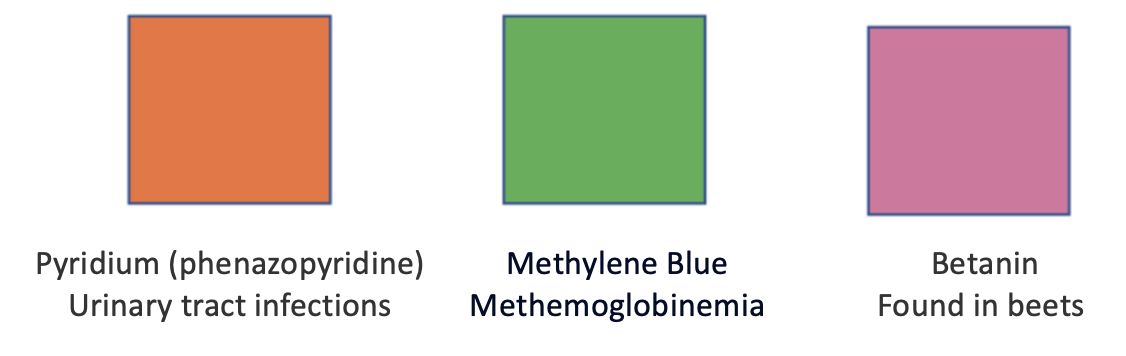
Three odd urine colors caused by certain drugs and foods will scare the hell out of you. There are many others.
So, let's answer the age-old question, "Why is pee yellow." This urgent matter can be answered by chemistry, specifically conjugated double bonds and a newly discovered enzyme.
The explanation requires – obviously – a little chemistry, which means that it is time for another...
Dreaded Chemistry from Hell®! Have a seat and enjoy!

Steve (left) bemoans a condition that isn't entirely different from that of middle-aged men, especially those who have a prostate the size of a deviled egg. Irving, as usual, is unmoved.
A new paper in Nature Communications reports on a previously unknown enzyme, which the authors named bilirubin reductase, aka BilR. This enzyme is responsible for reducing (adding hydrogen) bilirubin, a degredation product of heme (Figure 1) to form urobilinogen. Although you might wonder what the hell this has to do with pee color, hang on (Figure 2).
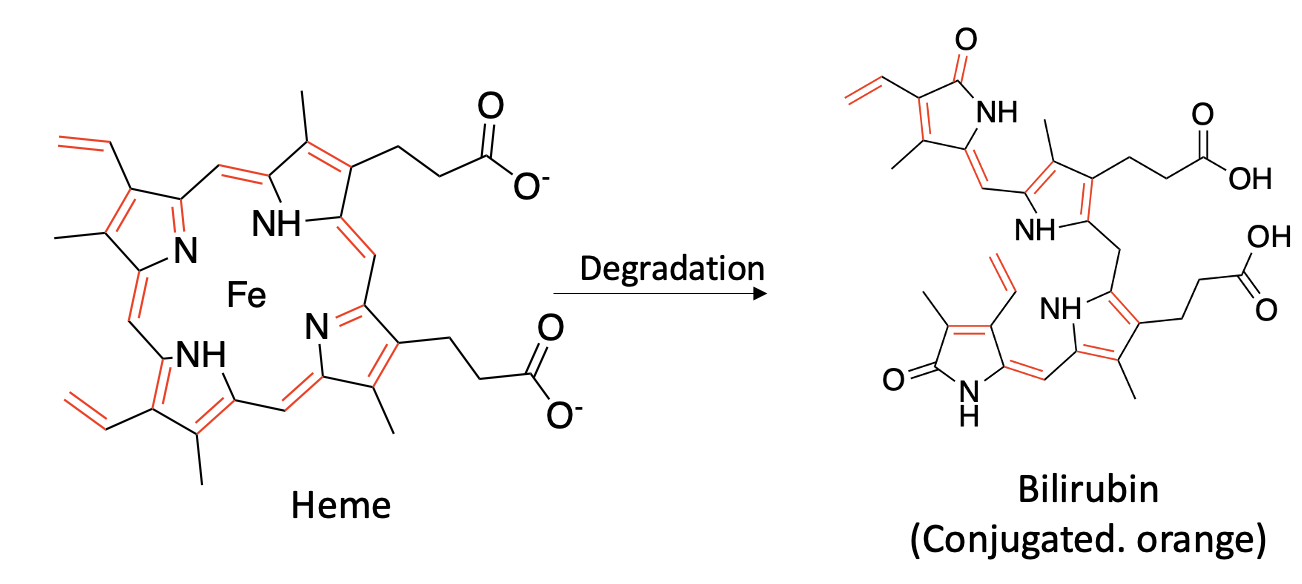
Figure 1. Heme, a precursor to hemoglobin (1), is degraded to bilirubin, which is part of the process of destroying and removing old red blood cells. Note that heme and bilirubin are conjugated molecules; they contain alternating single and double bonds (marked in red and black). For reasons you don't want to know, this typically gives organic molecules color.
Here's a familiar example of the effect of conjugation on color:
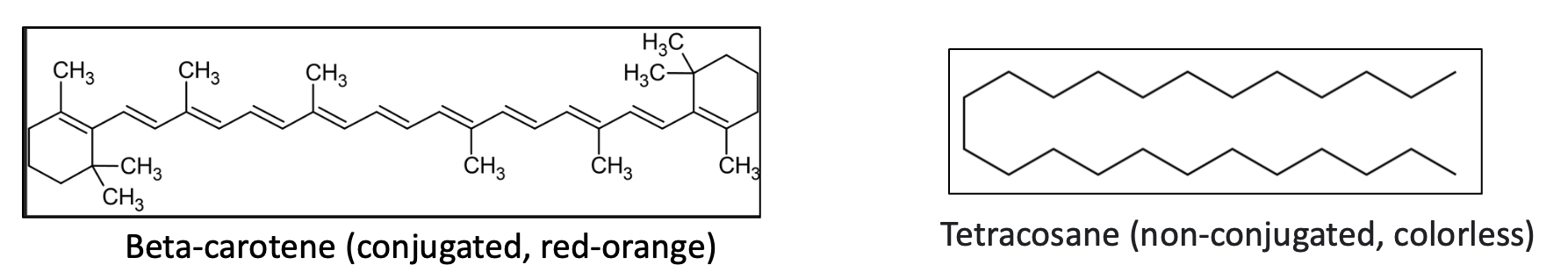
Both beta-carotene and tetracosane are hydrocarbons (they contain only hydrogen and carbon). Beta-carotene (note the alternating single and double bonds) is responsible for the colors of many fruits and vegetables, while saturated (fully hydrogenated) tetracosane – not an exact match but close enough – (or mineral oil, a long chain of CH2 units, others) is colorless. If you were to take beta-carotene and hydrogenate it so that all the double bonds were replaced by CH2 units it would also be colorless.
What does this have to do with pee color?
Pee-lenty. As shown in Figure 2, the group that published the Nature Communications paper discovered a new enzyme that converts bilirubin, which has multiple conjugated bonds in a row, giving it a yellow color into urobilinogen, which is colorless. The blue ovals designate the double bonds that have been reduced in the reaction. This disrupts the single-double bond conjugation and "kills" the color.
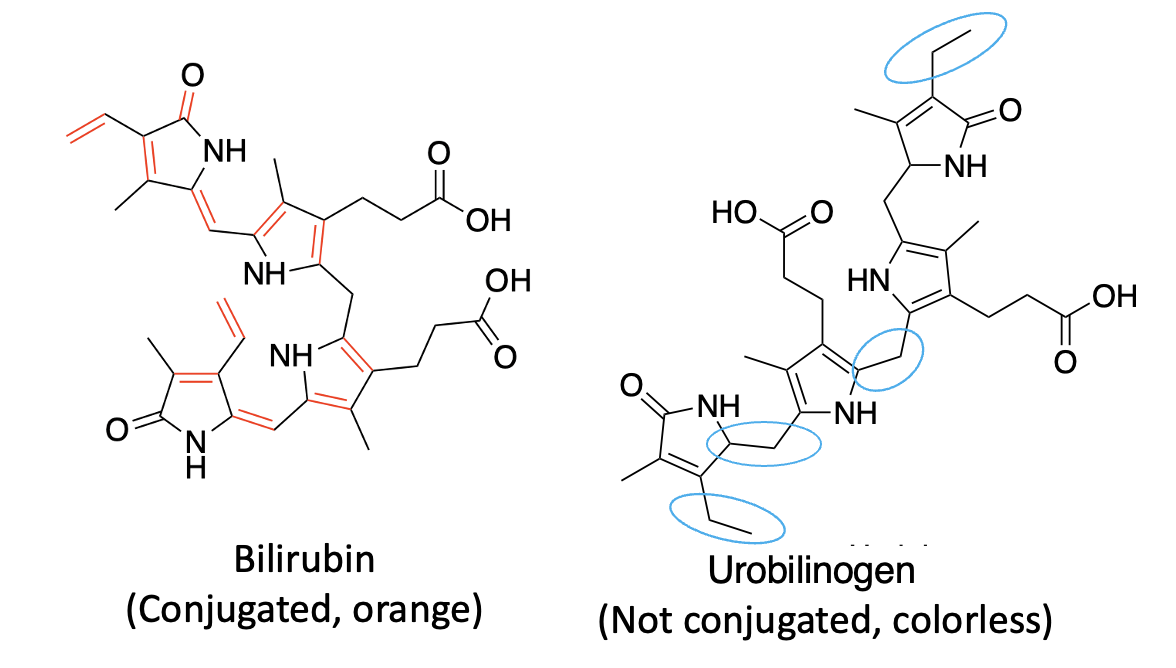
Figure 2. Bilirubin reductase converts bilirubin to urobilinogen (I don't know you, but I'm getting mighty sick of chemicals beginning with "ur." The blue ovals indicate the carbon-carbon double bonds that are reduced in the process.
But we're not done
In honor of the Geneva Convention, I will finish this torment soon.
Once formed, urobilinogen, which is unstable, is readily oxidized, either chemically or by gut bacteria, to form urobilin (Figure 3). Note that, unlike urobilinogen, urobilin is (like bilirubin) a conjugated molecule with alternating single and double bonds (green oval). In other words, BiIR coupled with subsequent oxidation of urobilinogen makes the molecule conjugated again. This process forms urobilin, which is yellow and stable enough to pass through the kidneys and be excreted. Hence, yellow pee, albeit by a somewhat convoluted (some may argue tedious) process is the result of the reduction of bilirubin to urobilinogen followed by oxidation to urobilin, the chemical responsible for the color of pee.
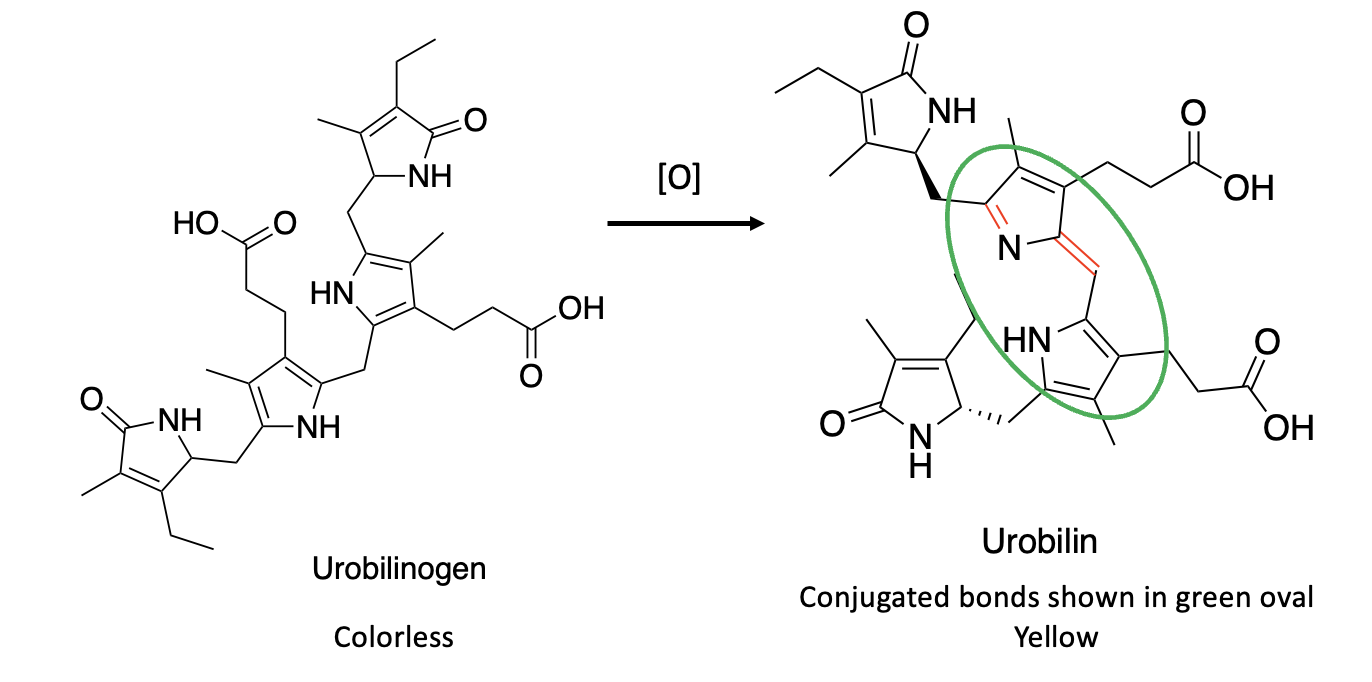
Figure 3. Bacterial oxidation of urobilinogen to urobilin.
Isn't it interesting that we have been peeing for 10 bazillion years, yet it was not until 2024 that we knew why the stuff is yellow?
Chemistry is some crazy s###
Finally, for those of you who are deranged enough to have gotten this far, it is my doodie duty to finish this wretched story. But in some ways, it even borders on interesting, especially for those who may be inclined to favor excretory trivia.
If Figure 3 didn't have you reaching for the ricin, keep going. This is actually kind of cool. The essence of the Nature Communications paper is the discovery of bilirubin reductase, aka BilR, which provides a previously unknown explanation of why urine is yellow.
Why stop here? It would be just plain wrong not to add additional excreta to this pantload of an article. It also provides a fine example of how minuscule chemical changes in a molecule can affect its properties. Figure 4 (below) completes the intestinal chemistry lesson.
As I explained earlier, when reduced by BiIR, bilirubin loses its color. This applies equally to the brown color of feces. Note the carbon atom in the pink circle. In urobilinogen, it is saturated (CH2), which "disconnects" the conjugation pathway of alternating single and double bonds causing the loss of color. However, this reduction process is never complete. Some bilirubin will make it to the colon, at which point another reaction will partially reduce the substance to give stercobilin – the heme degradation product that gives feces their brown color.
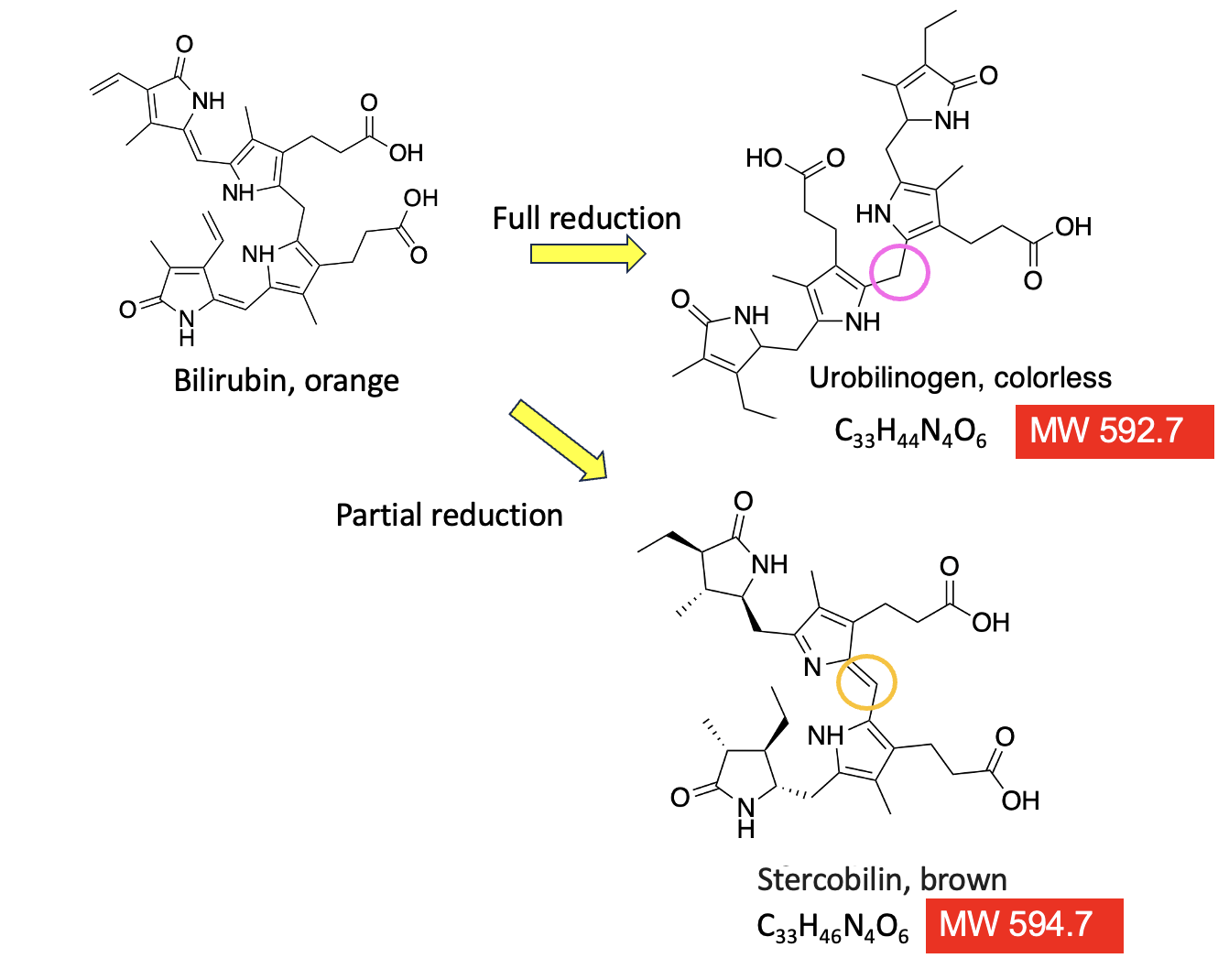
Figure 4. Urobilinogen and stercoblin are nearly identical molecules, with the exception of two little hydrogen atoms.
Look at the two molecules, each derived from heme. They are almost indistinguishable. Stercobilin is urobilinogen minus two hydrogen atoms (note the almost imperceptible difference in structure, molecular formula, and molecular weight). The only difference is that the carbon atom that is denoted by the pink circle has no double bond connecting the two halves of the molecule. So, instead of being a non-conjugated (colorless) molecule, stercobilin is once again conjugated by virtue of that double bond. Instead of red, like bilirubin, stercobilin is brown.
The front and bottom line
Aside from some knowledge about what makes organic chemicals colored (or not), you never know when this information could come in handy. For example, when meeting your future in-laws for the first time and there is an uncomfortable lull in the dinner conversation, "Would you like to know why the front of my underwear is yellow and the back brown?" makes for a splendid icebreaker. And grounds for a pre-nup.
All done. I'm pooped.
NOTE:
(1) ChatGPT: "Heme is the iron-containing prosthetic group found within hemoglobin and other hemoproteins, while hemoglobin is the protein complex that contains heme and is responsible for oxygen transport in the blood."




