What is Ethylene Oxide?
As I discussed, ethylene oxide is a chemical found everywhere in the environment and produced naturally in our bodies. It is used in many industries and is particularly useful for sterilizing medical devices. It is found in urban air at levels ranging from 0.1 to 0.2 parts per billion (ppb) and is naturally found in the human body at levels equivalent to continuous exposure to 0.56 to 4.5 ppb in air.
The Ethylene Oxide Rule
In 2023, the EPA proposed new regulations for medical device sterilizing facilities that use ethylene oxide, finalizing that rule this March. The rule sets limits on 88 commercial sterilization facilities using ethylene oxide as a sterilizer. The EPA determined that 23 of these facilities pose high lifetime cancer risks to the surrounding communities and must reduce their ethylene oxide emissions based on the EPA’s 2016 Integrated Risk Information System (IRIS) on ethylene oxide. The rule requires facilities to install technologies, practices, and procedures within two years to reduce ethylene oxide emissions by over 90 percent, with a capital cost of $313 million.
The EPA used 0.0001 parts per billion (ppb) in the air, corresponding to a one-in-a-million lifetime cancer risk, as the acceptable level of ethylene oxide emissions in calculating the emission levels for each facility. This level is less than the amount of ethylene oxide produced in the human body.
IRIS is a centralized database presenting the EPA’s risk assessment conclusions on environmental chemicals. IRIS is not based on any legal or statutory authority, but, as in the case of ethylene oxide, it is often used by EPA as the basis for their regulations. A fundamental flaw of IRIS is that there is no practical ability to review and change IRIS assessments other than through litigation.
In 2016, the EPA revised the IRIS assessment for ethylene oxide, focusing on its cancer risk, changing the EPA’s cancer risk descriptor from “probably carcinogenic to humans” to “carcinogenic to humans.” This is the source of the 0.0001 ppb EPA calculation. This level was much lower than previously calculated and cited as proof that ethylene oxide was a much more potent carcinogen than previously believed.
It’s All About the Model
The American Chemistry Council (ACC), a trade group that represents more than 190 companies in the business of chemistry, and the Texas Commission on Environmental Quality (TCEQ) submitted comments (here and here) on the EPA’s proposed rule on ethylene oxide sterilization facilities. They identified several flaws in EPA’s 2016 IRIS cancer risk assessment that resulted in overly conservative values.
The EPA and the TCEQ risk assessments are similar in the following ways:
- They both used United States-based worker cohort studies from the National Institute of Occupational Safety and Health (NIOSH) to derive their risk-based values.
- They both determined mutagenicity, the ability to cause mutations, as the mechanism causing cancer, using a linear non-threshold model (LNT) to assess cancer risk.
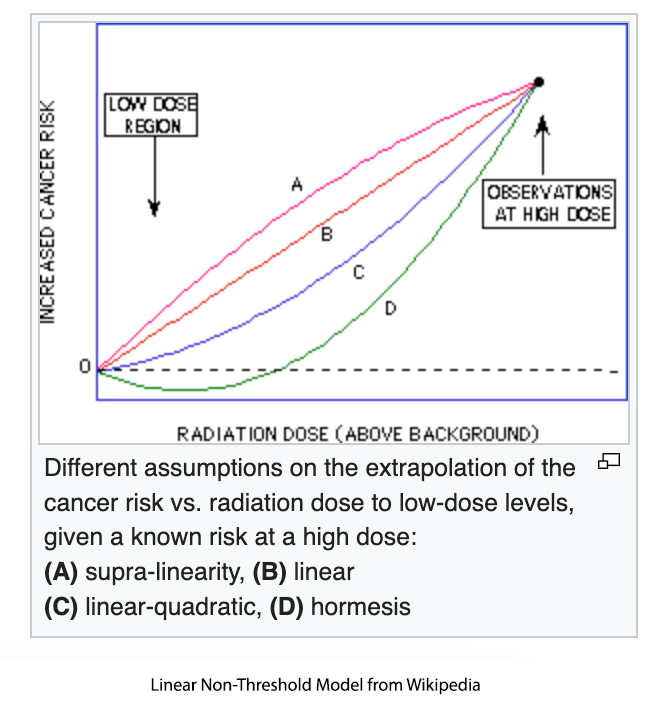 However, there are different mathematical models for LNT. The EPA used a supra-linear model that assumes low doses of ethylene oxide are more potent than high doses in causing cancer, a very questionable assumption. There is no biological data that supports the use of this model because the supra-linear model significantly over-predicts risk and is rarely used in risk assessment.
However, there are different mathematical models for LNT. The EPA used a supra-linear model that assumes low doses of ethylene oxide are more potent than high doses in causing cancer, a very questionable assumption. There is no biological data that supports the use of this model because the supra-linear model significantly over-predicts risk and is rarely used in risk assessment.
Texas used the linear model, which draws a best-fitting straight line from the high dose-response data down to the low doses. This model is more realistic and more frequently used in risk assessment. Even when determining a one-in-hundred thousand-lifetime risk, Texas estimated a cancer risk of 2.4 ppb, 24,000 times lower than EPA’s estimate.
Which model is best?
The data supports the use of the TCEQ cancer assessment over EPA’s 2016 IRIS assessment:
- When real-world data from the NIOSH worker studies are fitted to both models, the TCEQ model predicted the actual numbers of deaths much closer than the EPA model (which predicted deaths two and a half times greater than what actually occurred).
- The TCEQ assessed newer data on ethylene oxide (from 2016 to 2020), while EPA’s IRIS assessment was limited to data up to 2016.
- The TCEQ model calculated an acceptable air concentration of 2.4 ppb, which is within the range of ethylene oxide normally produced in the body (corresponds to the 75th percentile in nonsmokers), while the EPA concentration of 0.0001 ppb is 5,600 times below the lower part of the range of ethylene oxide produced in the body (0.56 ppb).
- The EPA-acceptable concentration of 0.0001 ppb is less than the background incidence of ethylene oxide found in air (0.1 – 0.2 ppb).
This is the first of three EPA rules dealing with ethylene oxide emissions. This rule is the smallest in scope, and there is already litigation over using IRIS in another of the rules. This underscores why there needs to be a process change for IRIS so that public input and sunlight are built in at the beginning before litigation becomes the only option available to bring changes.
It is not surprising that an Administration pushes its policy agenda. But it’s unacceptable for an Agency that should be driven by the best data and latest science to stay with a flawed assessment based on out-of-date data, particularly when they will impact millions of people.




