Until the arrival of the GLP-1 medications, bariatric surgery was the most effective means of reducing and maintaining weight loss. Yes, other methods work, but surgical intervention, the one-and-done, achieved the best results, and in several studies, as we now see with GLP-1’s, reducing mortality risks for cardiovascular disease and diabetes.
For many, given a choice between the blade and the pill, all you needed to indicate was how many pills and when to start. The shift back to non-operative management threatens a very large surgical practice. In a recent issue of JAMA Network Open, the surgeons made their economic case. But before jumping to their analysis, here is a bit of background courtesy of HealthSystemTracker, a joint venture of the Kaiser Family Foundation and the non-profit Peterson Center on Healthcare.
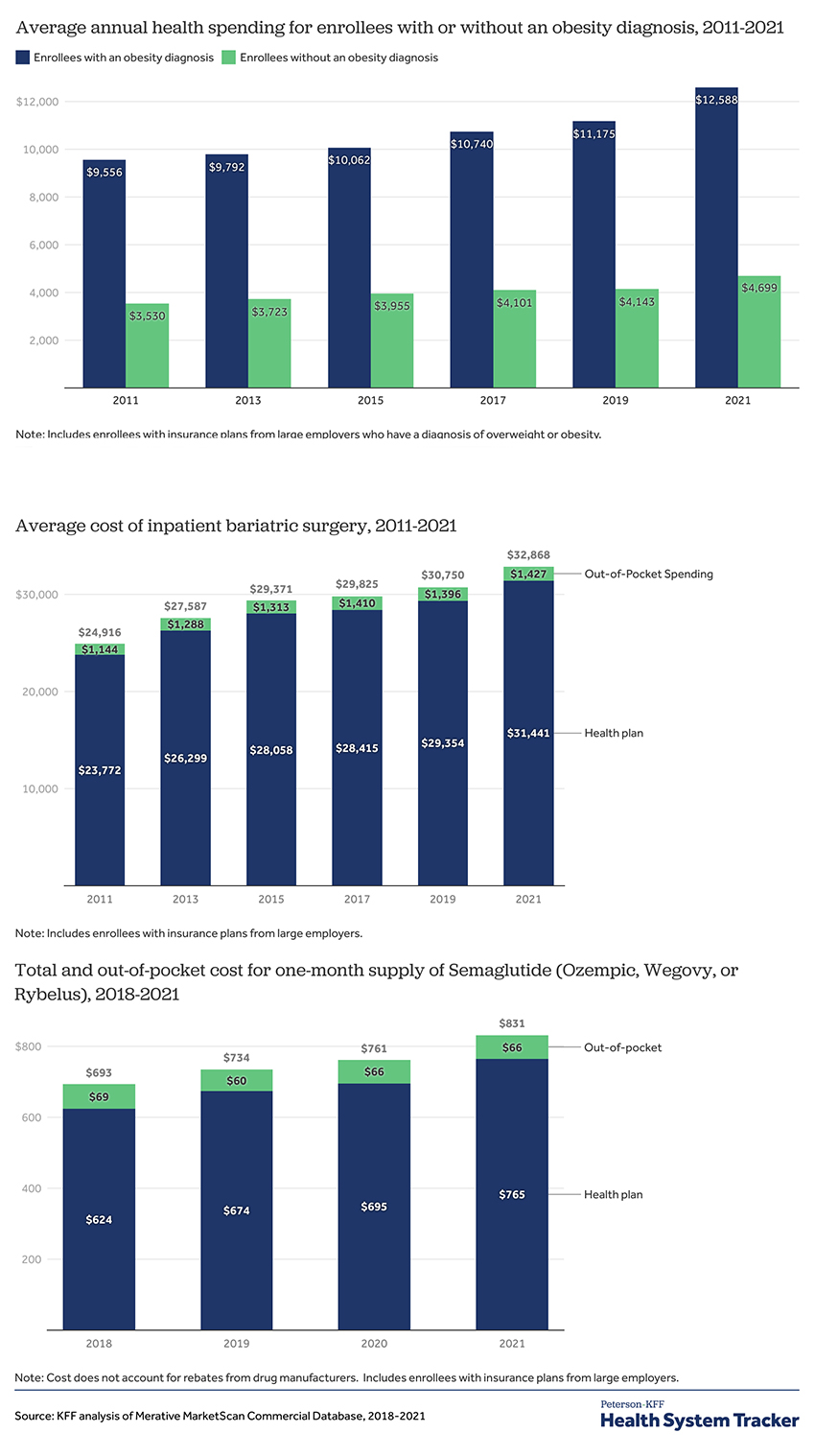
Three Graphs Tell the Tale
- Since 2011, the share of the US population with obesity, the primary indication for surgical or medical intervention, has increased from 28% to 34%.
- The spending on individuals with obesity is roughly 2.5 fold greater than those individuals that are not obese – both have increased by essentially the same percentage, 33%.
- Out-of-pocket expenses for the obese have increased by about 12% more than for those not obese.
- The cost of inpatient bariatric surgery, performed for 84% of patients, has risen by 33%, while outpatient surgery, for the remaining 16%, has been increased by only 7%.
- The annual cost to insurers for GLP-1 seems to be about $9,180, and patient out-of-pocket costs are roughly $792. This is an approximately 20% increase from the prices when the medications were introduced in 2018. These numbers do not reflect rebates, discounts, and all the other opaque costs of medications. [1]
Surgery – the Better Economic Alternative?
This brings us to the current study, which is simply an economic model where we can argue about the assigned values. The model considers a “45-year-old patient with class II obesity” (Class II reflects about 9% of our population) treated with semaglutide or a bariatric operation (endoscopic gastric sleeve gastroplasty [2]) over a 5-year time horizon. To make the model more “life-like,” the researchers included a percentage of adverse events; for the semaglutide group, an intolerance to the medication; for the surgical group, recognized surgical complications and failure of the initial procedure were modeled as requiring a revision of the surgery.
For the purposes of our discussion, two of those assigned values are important. The cost of semaglutide placed at $13,618 and the cost of surgery at $16,360. That makes semaglutide more costly than the KFF numbers by roughly $3600 and the surgery less costly than the KFF number by $6,300 for outpatient surgery and over $16,000 for inpatient surgery.
Quality-adjust life years (QALY) – the model gave a bump in quality for the changing BMI; they did not penalize for stopping medications but extracted a penalty for surgical complications. At the end of five years, there was
- A slight increase in QALY’s, 21 days, in favor of surgery.
- A slight decrease in the reduction of BMI again in favor of surgery (BMI 31.7 for surgery, 33 for semaglutide.
- A cost savings from surgery of $33,583 vs expenditures for semaglutide. These savings did not become apparent until the second year, given that surgical costs were front-loaded into the beginning of care and the cost of semaglutide was continuous.
Of course, it should be readily apparent that using KFF’s costs might tip the balance, at least economically. Reducing the cost of semaglutide by $3600 annually and bumping up the surgical costs to reflect outpatient surgery results in a cost, still in favor of surgery of $11,283. Suppose we substitute the more expensive and more frequently performed inpatient surgery that difference now favors medical management by roughly $417. This makes the conclusions of the researchers uncertain.
“It was concluded that for all parameters, the cost of semaglutide must be decreased by at least 3-fold to cause any change in the strategy preference.”
A few concluding thoughts. First, it is essential to recognize how the assignment of costs, let alone the assumption concerning adverse complications, will alter the cost-benefit analysis. Often, this is managed by sensitivity analysis, where these variables are changed across a larger range to determine if a new conclusion can be drawn. In this case, only a reduction in the cost of semaglutide made a difference – partly because surgical care in the model was consistently undervalued and semaglutide costs overstated.
Second, cost savings would not be apparent for two years or fully realized in the model for five years. Insurance companies that experience significant turnover in beneficiaries have no reason to pay the large upfront costs of surgical intervention when they are far less likely to reap the later benefits. JAMA reports that over 20% of beneficiaries change their commercial insurance annually.
The debate between surgical and medical approaches to weight loss management is multifaceted, encompassing clinical efficacy and economic feasibility. As highlighted in the analysis, the cost-effectiveness of interventions hinges on nuanced factors and assumptions. Moreover, the delayed realization of cost savings underscores the complexities of balancing upfront expenditures with long-term benefits.
[1] For the econ types, these increases disappear when accounting for inflation. Health spending has decreased by about 8%, and inpatient surgery by a similar amount. Outpatient surgery showed the greatest decline by about 34% and medication by 4%.
[2] Using an endoscope, so there are no incisions, the surgeon reduces the size of the some by creating a tube (the sleeve). The reduced stomach capacity leads to earlier feelings of fullness and reduced food intake.
Source: Semaglutide vs Endoscopic Sleeve Gastroplasty for Weight Loss JAMA Network Open DOI: 10.1001/jamanetworkopen.2024.6221




