I bet you didn't know that the ancient Greeks manufactured purple dye (1) about 3,600 years ago during the Early Bronze Age. This was a surprise to many, until 2017 when an archaeology team discovered a building on the Greek island of Aegina Kolonna (also known as Kolonna). The building is where a rare and valuable purple dye called Tyrian Purple (2) was extracted from mollusk shells. A recent article published by Lydia Berger and colleagues from the University of Salzburg, using modern analytical techniques, provides evidence that the dye was indeed manufactured on Aegina Kolonna as early as 1,600 BCE. by examining the component of the dyes used to make the purple.
The Late Bronze Age settlement of Aegina Kolonna housed workshops for the on-site production of purple dye.
This discovery offers a fascinating glimpse into the dye technology of the time. Here's a brief look at some of the interesting color chemistry associated with Tyrian Purple. (Figure 1)

Figure 1. Top row: (Left) The island of Aegina in the red circle (Image: Wikimedia Commons). (Center and right) the snail pit – hardly the place for a luxury vacation – before and after excavation in 2017. Bottom row: (Left) The pottery fragments discovered by the group. (Right) Purified pigment. Credit: L Berger, PLOS. (Bottom row)
What is Tyrian Purple?
There is no universal answer to this question. Here's why. The group performed HPLC-UV analysis of the Tyrian purple samples, which revealed something a bit strange: high levels of purple monobromoindigotin (MBI ) and moderate amounts of dark red dibromoindigotin (DBI), lighter red 6,6-dibromoindirubin (DBIR) and blue indigotin (IND). From this pattern the scientists were able to confirm that the dye was manufactured in Aegina.

The primary colors identified by the University of Salzburg group. (Top) DBI (dark red), MBI (purple). (Bottom) Indigo (blue), DBIR (light red). But the term Tyrian purple is often used interchangeably with dibromoindigo, which isn't even purple (!)
A Tyrian Mystery?
Please bear with me. If you look for the chemical name for Tyrian Purple you'll find this: 6,6'-dibromoindigo (DBI), a chemical that is (obviously) related to indigo, a blue dye that has been used for at least 6,000 years. A couple of "colorimetric" problems: Tyrian purple is actually a mixture of at least four pigments, only one of which (MBI) is purple. And 6,6'-dibromoindigo, which is the "official" name for the dye is dark red, not purple. Huh? Simple answer? Purple + light red + dark red + blue = Tyrian purple.
Structure-Color Relationships
Anyone with even a passing knowledge of medicinal chemistry will be familiar with structure-activity relationships (SAR) – the cornerstone of drug discovery. This technique involves making small, incremental changes to an early compound of interest (often detected in a large-scale screen but rarely not good enough to make it into clinical trials) and looking for (hopefully) improved compounds.
This technique helps chemists zero in on the "good" and "bad" parts of the molecule as they interact with the biological target. The next round of compounds – made by medicinal chemists or robots – are tested in biological screens. The results can then begin another round of SAR to build on the first findings. The same basic principle applies to dyes. Making derivatives of a dye like indigo (below) results in an impressive array of colors.
Substituted indigo compounds can have very different colors
Indigo (left) and dibromoindigo (right) differ only by a bromine substituent on the benzene rings. Yet this is enough to change the electronic properties of indigo, turning it from blue to dark red. (If, for some ungodly reason, you want to dig deeper (hate that phrase) I have written about conjugated single and double bonds, which frequently give organic molecules color.)
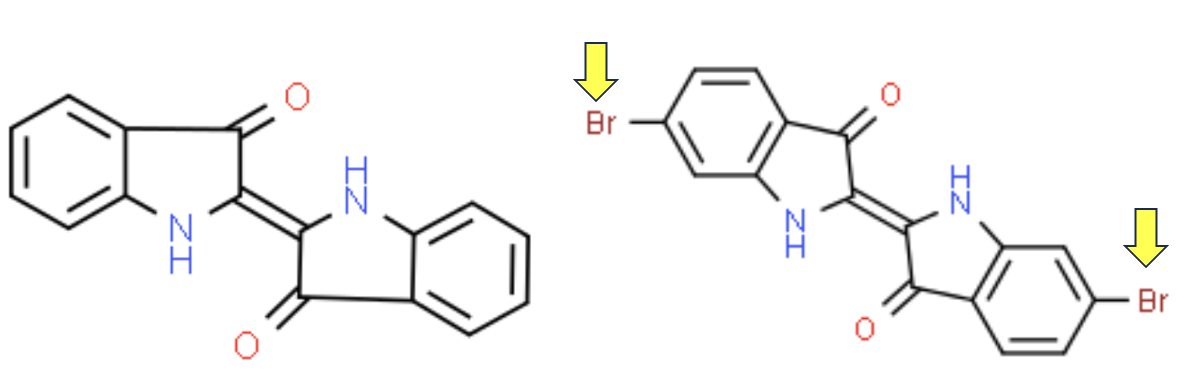
Indigo (left) and Tyrian Purple (right) differ only by the substituents on the benzene rings (yellow arrows), but their colors are quite different.
Can different substitutions produce other colors? Oh yes.
An large number of indigo dyes have been synthesized and they are not all shades of blue or red. By substituting other functional groups instead of bromine the colors change radically. Below are some examples.

Indigo derivatives may or may not be indigo.
Conclusion
It's pretty cool that the analysis of the chemical dyes in two pottery fragments filled in a piece of Greek history, telling us that the ancient Greeks were perhaps better chemists than we thought.
That's all I have. Perhaps, if you find yourself in a snail pit in Aegina perhaps this will come in handy, although I doubt it. At the very least I hope you enjoyed the pretty colors.
(1) The Greeks manufactured very little Tyrian purple; they weren't especially good at it. But the Phoenicians were. They shipped the due to Aegina, which was a major trading port at the time, and the dye was sent then sent to other locations.
(2) Tyrian purple was both rare and valuable. It was used by the wealthy, the church, and high-ranking military officers.




