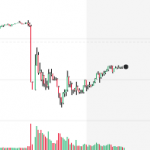
Preliminary data, obtained from a randomized clinical trial of remdesivir in China, look bad. Maybe even very bad. But this doesn't mean that the drug won't be shown to be useful in other trials. Nonetheless, not good news.
Drugs & Pharmaceuticals
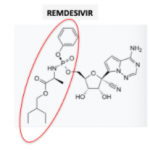
Remdesivir, the most promising anti-coronavirus drug at this time is no fun to synthesize. But Gilead, the drug's maker, is synthesizing a whole bunch of it. Does this tell us anything about whether the drug works? Maybe.
So much news, so much confusion and so many questions – especially those around what different terms mean. What exactly is a therapy for COVID-19? Is it a cure, or something else, like a vaccine? To help sort it out, we prepared this summary; it may help a bit. And to go with it, a riddle: What do you call anti-vaxxers once a coronavirus vaccine becomes available?
While recognizing the Centers for Disease Control’s missteps in handling this epidemic, I also understand that the agency could still provide critically important leadership in getting us out of this mess. However, its botched attempt at providing testing for public health labs around the U.S. was clearly a major roadblock to establishing the kind of robust testing we would have needed early to contain the outbreak. I miss the CDC I used to know.
Early clinical trial results from Gilead show that its antiviral drug, remdesivir, has promise in treating patients with severe COVID-19. Though there are major caveats, there is good reason for cautious optimism.
Dr. Derek Lowe, arguably the finest and most influential chemistry blogger in the universe, has put together an excellent summary of the complex and confusing clinical data of hydroxychloroquine, which he published recently in his blog in Science and Translational Medicine. We thank Derek and AAAS for allowing us to reprint this important article.
Despite study after study, and the writings of expert after expert over the last 25 years, as a society we've failed to provide for our own security in the face of a potential public health threat. We have failed to supply and maintain our strategic national stockpile, we have consistently underfunded our public health infrastructure and we have underfunded our hospitals' preparedness. That's how we got to where we are today.
The world anxiously awaits while clinical trials of remdesivir are in progress. The drug failed to stop Ebola. Does this mean it will also fail to stop coronavirus? No. According to a new study in the Journal of Biological Chemistry, the drug should work better. Here's why.
In the frantic fight to get an effective antiviral into the hands of a terrified world, there's a new kid on the block. This one is called N-hydroxycytidine and it's rather interesting. NHC is a potent inhibitor of coronavirus replication in cells, it's really easy to synthesize and it'll protect you from the virus. (That is, if you're a lab rodent.)
Everyone take a deep breath and relax. During these crazy times, people are making all kinds of wild predictions about what drug or vaccine will work. Dr. David Shlaes takes a sobering look at the chances for any of these therapies to work. It's not as easy as you'd think. We should all lower our expectations a bit.
For a simple drug, there sure is a lot of controversy surrounding hydroxychloroquine -- a malaria drug that's one of a handful of repurposed drugs being evaluated as potential anti-coronavirus treatments. However, hydroxychloroquine (HCQ) doesn't look especially promising. Dr. Katherine Seley-Radtke, Professor of Chemistry and Biochemistry at the University of Maryland-Baltimore County, explains.
Those are the words of New York Gov. Andrew Cuomo, describing the "twist of fate" that finds us awaiting personal protective equipment, ventilators and pharmaceuticals manufactured -- yes -- in China.