With antibiotic resistance a growing threat, scientists are on the hunt for new ways to treat bacterial infections. One of these, called phage therapy, uses a special kind of virus that only infects and kills bacteria. (These viruses are called "bacteriophage" or simply "phage.")
The original idea for this therapy is actually quite old. It was pioneered by Félix d'Herelle in the 1920s (and is still used in Eastern Europe today) but it fell mostly out of favor with the advent of antibiotics like penicillin. However, with antibiotics becoming less effective today, scientists are increasingly turning to unconventional treatments.
Acinetobacter baumanii, often referred to as "Iraqibacter", gained notoriety in recent years due to its causing wound infections in soldiers returning from Iraq and Afghanistan. And like so many bacteria these days, it is resistant to multiple antibiotics and is easily transmitted in hospitals.
To address the problem posed by A. baumanii, a team of American military scientists turned to phage for assistance. Just like with antibiotics, bacteria can become resistant to phage. So, instead of using one phage to target A. baumanii, the team created a very clever cocktail of five wild phages.
The team created wounds on the backs of mice and infected them with bioluminescent (glowing) A. baumanii. Then, the mice were injected with a control solution (PBS), a one-phage solution (AB-Army1), or the five-phage cocktail (AB Cocktail). Bioluminescence was monitored, which indicated roughly how many bacteria were present and how far the infection had spread, and a heat map was generated. (See figure.)
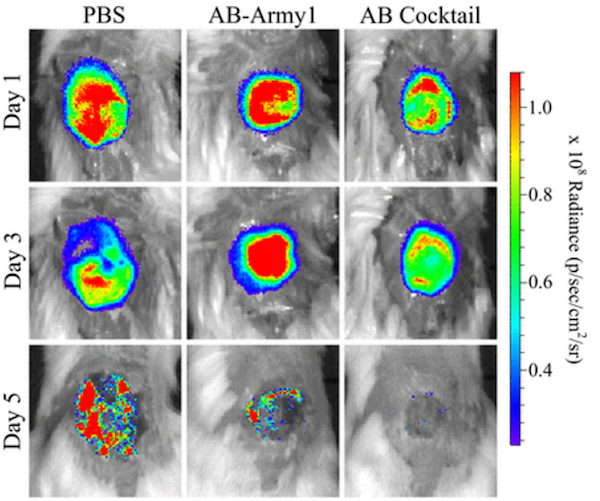
As shown, mice that were given a control solution had a difficult time clearing the infection. On the other hand, mice given the five-phage cocktail cleared the infection after roughly three days. Additionally, these mice lost less weight than the other mice during the course of the infection.
The phages in the cocktail used different mechanisms to kill the bacteria. The AB-Army1 virus, which was a part of the cocktail, did not kill of all the bacteria. Instead, it disarmed them. Some bacteria, like A. baumanii, produce a slimy capsule that inhibits the immune response. Because the AB-Army1 virus likely binds to a protein found in this capsule, it infected and killed only the bacterial cells which produced it, leaving the capsule-free bacteria as survivors. But these "naked" bacteria were then exposed to assault by the other four phages, which infected and subsequently caused them to explode.
The upside and downside to using phage therapy is that the viruses are extremely specific. The upside is that phage will only kill a very specific type of bacterium, whereas antibiotics lay waste to many different bacteria, including friendly ones. The downside is that the phage are too specific. In fact, when the authors tested their five-phage cocktail on 92 clinical isolates of A. baumanii, it only killed 10 of them. Thus, the authors conclude that phage therapy must be highly personalized. Different cocktails would be required to treat different A. baumanii infections.
As daunting as that sounds, it could be overcome if bacterial screening technology improves. Once that occurs, finding the viruses is the easy part. The researchers obtained all of the phage in their cocktail from sewage water.
Source: James M. Regeimbal et al. "Personalized Therapeutic Cocktail of Wild Environmental Phages Rescues Mice from Acinetobacter baumannii Wound Infections." Antimicrob. Agents Chemother. 60 (10): 5806-5816. Published: October 2016. doi: 10.1128/AAC.02877-15
(Image: Phage via Shutterstock)