
Once in a while (just for yuks) I'll write about some of the hideous chemicals that we chemists have to use now and then despite the fact that they are not only a pain in the a##, but also mighty dangerous.
For example, as a new grad student, I needed to use a horror show called t-butyllithium, an organometallic (1,2) chemical reagent that is so violently unstable that it comes in a hexane solution in a sealed, airtight bottle with a rubber serum cap. Any air that might remain in the bottle is flushed out with nitrogen or argon (3). When you need to use this bad boy you don't just unscrew the cap and pour it out (4); it is withdrawn by syringe in a setup shown in Figure 1. Even using all these precautions, whenever the syringe is withdrawn from the bottle there is a tiny amount at the tip of the needle which will always catch fire just from being exposed to air. Lovely.
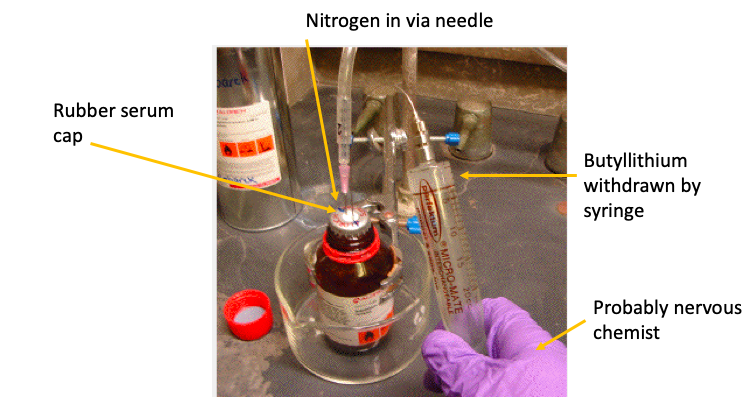
A typical setup for transferring pyrophoric (catches fire) organometallic reagents. Original photo: Oregon State University
Even when proper safety techniques are used there is always a risk of a fire. In 2009 a research assistant at UCLA died when her clothing was set on fire from an accidental t-butyllithium spill. (See Butyllithium — Something You Really Don't Want To Mess With.)
Here's a video of some lunatic shooting the stuff into the air:
https://www.youtube.com/watch?v=7aJitlPFWOs
What could possibly be worse than this? One could make the argument that there is such a thing – a truly hideous reagent called diazomethane. Of course, taste is subjective. Given the choice would you rather be incinerated or exposed to two different carcinogens and then blown up? Tough call. Here's the chemistry (Figure 1). 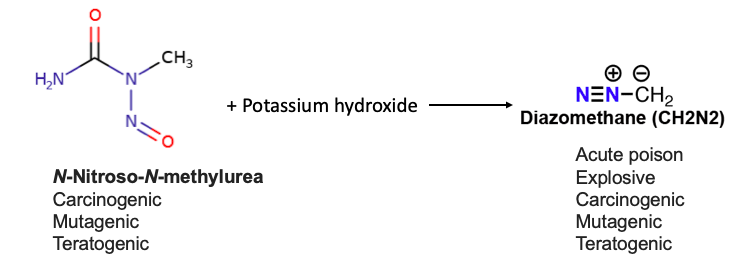
Figure 1. Conversion of N-nitroso-N-methyl (NMU) (yick) to diazomethane (double yick.)
The reaction itself is easy to perform; you just stir a flask containing a water solution of sodium or potassium hydroxide (the active ingredient in drain cleaners) and ether (water and ether are not miscible; two layers form, the ether will be the top layer). Then chuck in the NMU a little at a time. The diazomethane forms immediately and goes into the ether layer, turning it yellow. Then you have to do is remove the ether layer (on top) which contains the diazomethane. This can be easily done with a disposable pipet. Except...
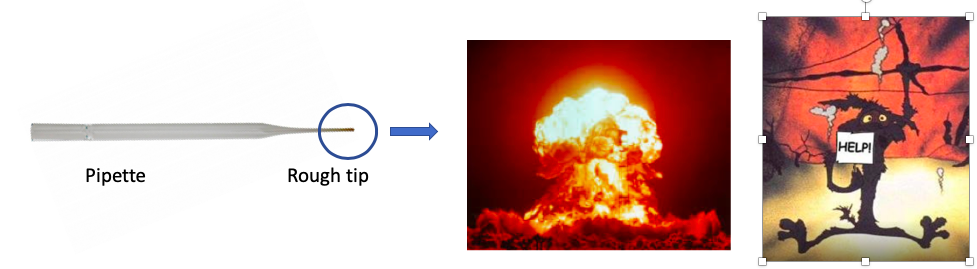
Oops. Forgot about the tip of the glass pipet. Images: Wikipedia, Twipu.com
When I said that diazomethane was explosive I wasn't kidding. If it touches any kind of rough glass that is enough to set it off. To avoid becoming part of a Road Runner cartoon you need to briefly hold the tip of the pipet under a flame so that it melts a little and smooths out the rough tip.
If this isn't bad enough, there are times when the diazomethane made like this isn't pure enough. Distillation is recommended, which is fine unless you are the one who has to distill the damn stuff.
Chemistry blogger Derek Lowe, the author of the wildly popular "Things I Won't Work With" series wrote about a full-fledged moron who somehow survived distilling diazomethane despite doing everything wrong.
[A colleague of mine attests that] when he was in grad school looked across the hall to see someone involved in making a goodly amount of diazomethane – in a large standard ground-glass-joint apparatus (5). Oh, dear. How the guy was going to get his collection flask off without running the risk of grenading everything?... As my friend watched in disbelief, the guy reached up to just twist the darn thing right off...and it was stuck.
(This is very bad. But not so bad that it can't get worse.)
A frozen joint – just the perfect time for it...my colleague swears that he then watched this maniac pick up a propane torch to sweat the joint loose. I believe that someone may have stopped him in time, but I think the teller of this tale decided to adjourn for lunch at some distant location right around then, so I can’t vouch for the outcome.
Derek Lowe, How Not To Do It: Diazomethane. April 30, 2008
In case you fledgling chemists out there haven't already changed your major to bio check out some of the stuff Wikipedia has to say about diazomethane.
- Diazomethane is toxic by inhalation or by contact with the skin or eyes.
- Symptoms include chest discomfort, headache, weakness and, in severe cases, collapse.
- Symptoms may be delayed. Deaths from diazomethane poisoning have been reported.
- In one instance a laboratory worker consumed a hamburger (Duh) near a fume hood where he was generating a large quantity of diazomethane, and died four days later from fulminating pneumonia.
- Like any other alkylating agent it is expected to be carcinogenic but such concerns are overshadowed by its serious acute toxicity.
- CH2N2 may explode in contact with sharp edges, such as ground-glass joints, even scratches in glassware.
- Glassware should be inspected before use and preparation should take place behind a blast shield. Specialized kits to prepare diazomethane with flame-polished joints are commercially available.
- The compound explodes when heated beyond 100 °C, exposed to intense light, alkali metals, or calcium sulfate. Use of a blast shield is highly recommended while using this compound.
Aside from that, it'll be smooth sailing. (7,8)
Here's an idea: Let's take a serious chemophobe, for example, Nick Kristof of the New York Times, who is so afraid of (harmless) bisphenol-A (BPA) that he won't touch cash register receipts, stick a lab coat on him, and let him help the propane torch idiot unstick the frozen joint. (6)
Boom.
NOTES:
(1) Organometallic chemicals all have carbon chemically bound to a metal. Most are unstable and decompose in water, sometimes violently.
(2) There are three different isomers of butyllithium that are sold. "t" (standing for tertiary)-butyllithium is the worst of them.
(3) Argon and nitrogen are chemically inert. Either gas can be used to protect air-sensitive chemicals.
(4) This isn't strictly true. You can pour it out – once.
(5) Ground glass refers to the joints of pieces of glassware that are made to form a tight seal. But when diazomethane touches ground glass it blows up. Here is what it looks like. Note the frosted areas where pieces of glassware connect to others.

(6) Despite knowing absolutely nothing about chemistry Kristof is cited by the ignoramuses at NRDC as a chemistry expert. Oh, my sides. See my critique of their hilariously bad video NRDC's Hitler-Pesticide Video Worthy Of Joseph Goebbels
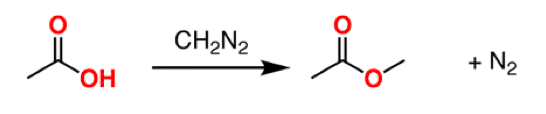
(7) An obvious question – why use this stuff if it is so dangerous? It's because diazomethane is the best reagent for converting carboxylic acids to methyl esters (a common and important transformation in organic synthesis).
(8) There are now safer alternatives to NMU and diazomethane as well as special distillation kits without ground glass joints. The "safer" replacement for diazomethane is called trimethylsilyldiazomethane aka TMS-diazomethane. But it's not perfect either. It is even more toxic than diazomethane itself. A chemist using it was killed in 2008.
