Glycated hemoglobin, aka, Hb1Ac, is an important measure of diabetes risk. The name sounds daunting, but the chemistry that forms it is quite simple. Even ACSH scientists might be able to understand this Dreaded Chemistry Lesson From Hell. At least on a good day.
With rare exceptions, the chemical reactions in our bodies are the result of enzymes that catalyze the process in question. Why? Because enzymes are designed to promote specific chemical reactions, this makes enzymatic reactions about 1 million times faster than the same reaction in the absence of the enzyme. In short, life depends upon enzymes.
But one of the few exceptions – a simple chemical reaction in the blood – is why we can estimate glucose levels in the blood over time. This provides a better look at whether we are heading for diabetes than a simple single-point blood glucose test, the results of which can vary widely.
That HbA1c (glycated hemoglobin) is formed by a simple sophomore-level organic chemistry reaction (more later) rather than a specialized enzymatic process makes sense (1). In fact, the reason that the HbA1c test works at all is that the reaction that forms it is not enzyme-catalyzed and is, therefore, very slow, allowing the test to provide an average measurement of blood glucose over three months.
What is glycated hemoglobin?
To answer this question, I must reluctantly call upon our old friends, Steve and Irving, who reluctantly answered the bell.

Steve (left) and Irving, the reluctant hosts of TDCLFH©, bored and cranky, are having an unproductive conversation. As if they have anything better to do?
The first part of the DCLFH is so simple that even our ACSH scientists, not the brightest bunch in the galaxy, could probably possibly maybe understand it. It's a very easy, common reaction: an amine with an aldehyde forming an imine (Figure 1).
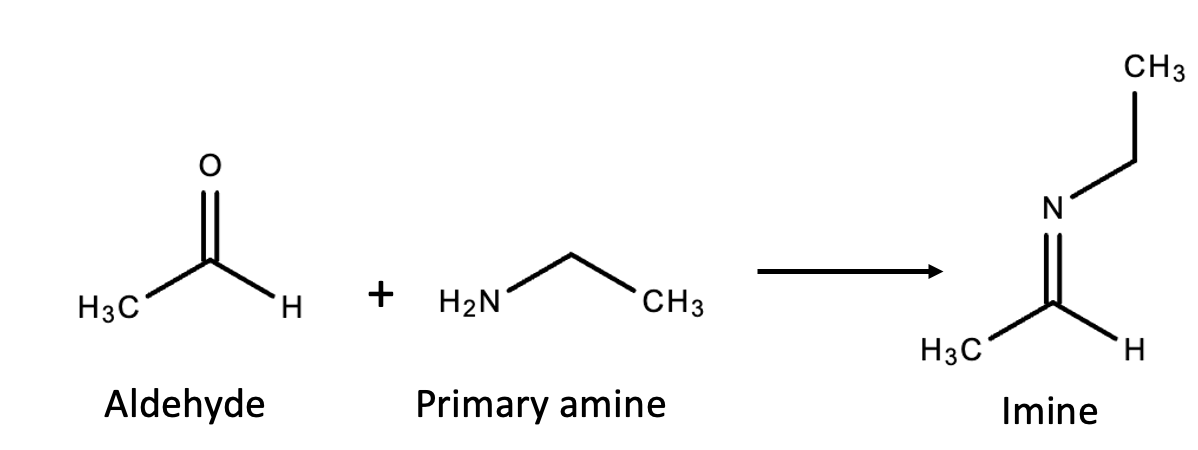
Figure 1. An aldehyde (pictured: acetaldehyde) reacts with a primary amine (pictured: ethyl amine) to form an imine. The byproduct is a molecule of water.
What does this have to do with glucose?
If you look at the chemical structure of glucose (left), you may wonder: "What is this idiot talking about? There's no aldehyde!" Answer from said idiot: Glucose exists in two forms (isomers) that are in equilibrium with each other. In its closed form, nothing can react with an amino (NH2) group on hemoglobin. But in the open form, voila – an aldehyde (red oval) makes a ghostly appearance (Figure 2).
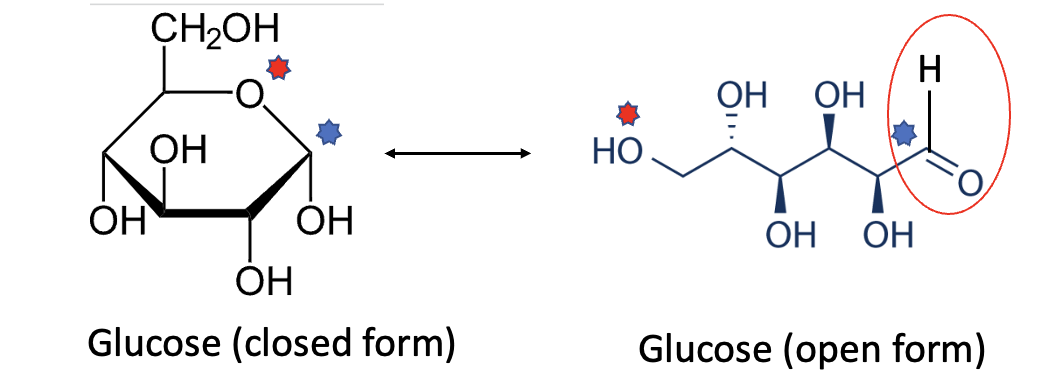
Figure 2. Glucose in its open (left) and closed (right) forms. The blue and red stars show the corresponding atoms in each form. (This is NOT easy to visualize without knowing some organic chemistry.) A red oval shows the aldehyde group in the open form.
Now we can make HbAc1
If you've gone through this torture, you might as well stick it out to the end, which is, mercifully, near. The same chemistry explains the formation of HbAc1, except that the amino group is a tiny part of the hemoglobin molecule.
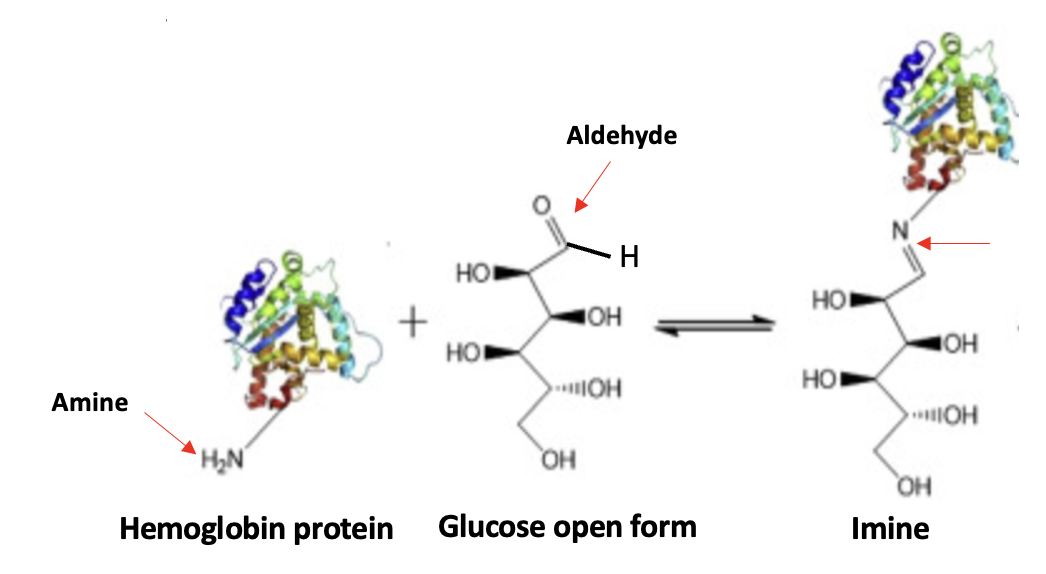
Figure 3. Proteins are made of 20 different amino acids. One of them (lysine) contains a side chain with an NH2 group. This enables proteins to form covalent (stable) bonds with small molecules like glucose. (This is similar to how the fentanyl vaccine works.)
Sorry suckers. We're not done!
Yeah, I know. Tricking readers into finishing this wretched article is not a great way to attract and keep a stable fan base. But if you've gotten this far, it's too late. I already gotcha. Joke's on you!
There is one more little piece to this puzzle. If you look at Figure 3, you'll see a double arrow between glucose and the imine. This means that the reaction is reversible, which also means that the imine is relatively unstable. So how will it stick around for months to act as a glucose surrogate? The answer is one last step, which happens spontaneously – a rearrangement. (Specifically, it is called a hydroxyimine-aminoketone rearrangement aka, an Amadori rearrangement (2) – perhaps the single most useless fact that you will ever encounter.
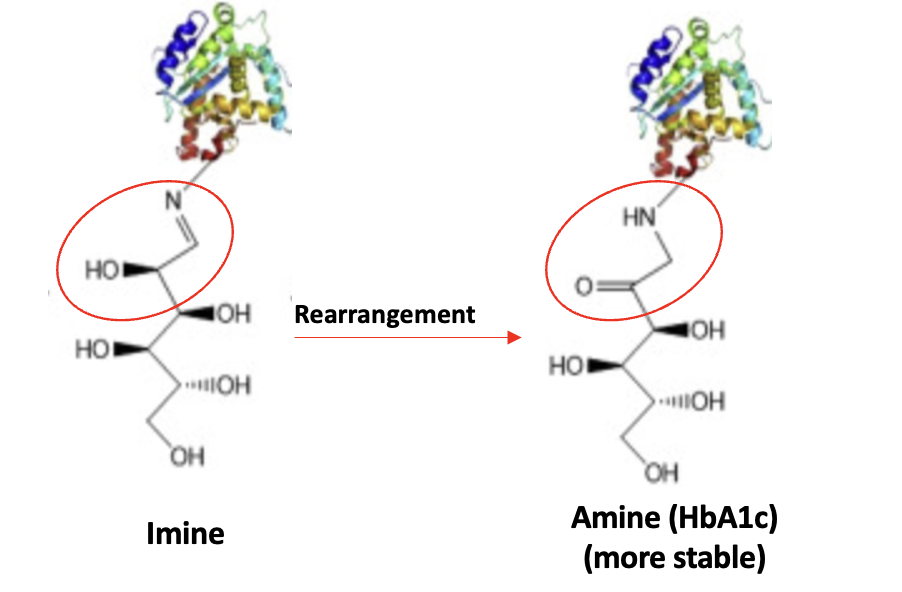
Figure 4. Finally. Rearrangement of the glucose-hemoglobin imine produces a more stable amine, which can be measured. I won't go into the details of how/why this works. And you won't be missing anything either.
Why this is kind of cool
The human body has evolved so that thousands of enzymes perform life-giving chemical reactions in a small fraction of a second. Without these enzymes, these same essential reactions would be far too slow to support life. Yet, this one non-enzymatic, simple reaction is medically helpful because it is so slow. Suggestion: This is not a good topic for dinner conversation. Unless you're dining with a group of chemists – an event that even Steve and Irving couldn't tolerate.
NOTE:
(1) Our enzymes are not floating around by accident. They are the product of millions of years of evolution. Yet, there is no enzyme to couple glucose to hemoglobin. This makes sense because our ancient ancestors did not eat a load of sugar or starch (or anything else), so glycated hemoglobin formation would have an irrelevant process.
(2) Thanks to commenter Jim Mitroka for reminding me of the name - something I forgot centuries ago.
