Last week, the FDA and CDC presented their recommendations for the newest round of COVID-19 vaccines. As with everything COVID, there are proponents and detractors, or, putting it another way, both knowledgeable experts and disinformation-spreading attention-seekers. The reality is that a group of experts made a judgment based on actual data. We discuss the evidence here so you can make your own informed decision.
The images come mainly from PowerPoint presentations to the CDC’s Advisory Committee on Immunization Practices. Links to our sources are at the end of the article.
Who's at Risk of Severe Disease?
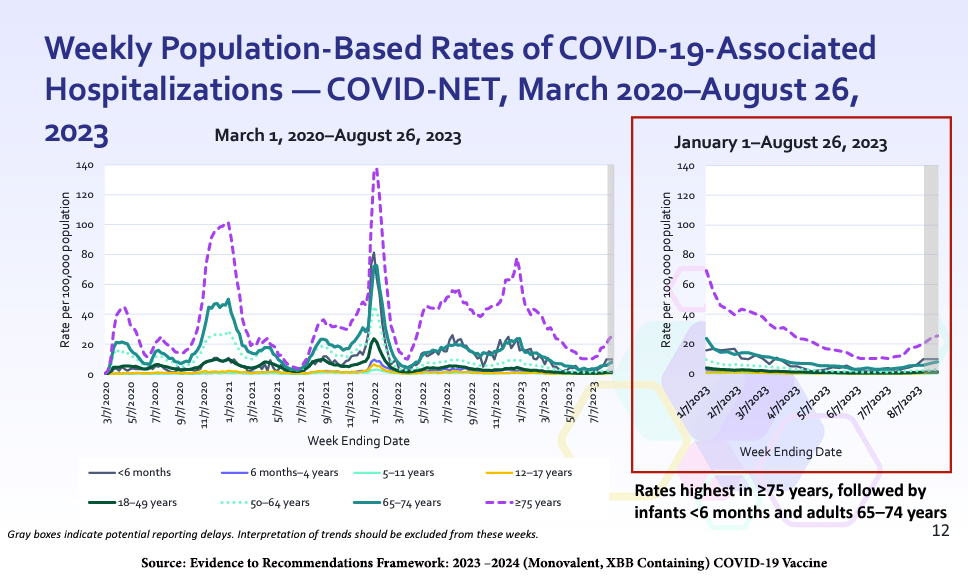
Current US hospitalization rates are highest for people ages 75 and up, with roughly 25 hospitalizations per 100,000 population; then, infants under six months and adults ages 65 to 74. The two latter groups experience about 10 hospitalizations per 100,000 population.
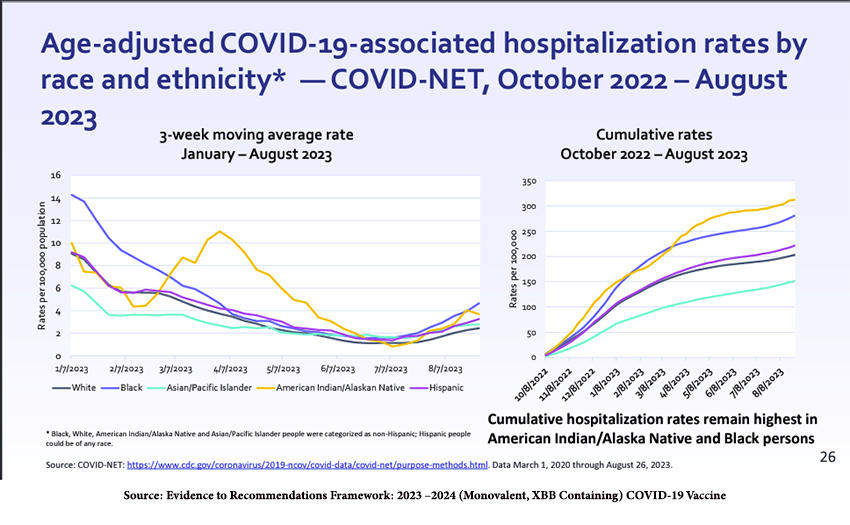
Hospitalizations continue to reflect racial and ethnic differences.
Among adults, the increasing number of comorbidities -- the simultaneous presence of medical conditions, such as obesity, diabetes, cardiac or renal disease, along with COVID-19 infection --increases the risk of hospitalization, the need for mechanical ventilation, and death. The presence of comorbidities demonstrates both a geographical and ethnic/racial distribution.
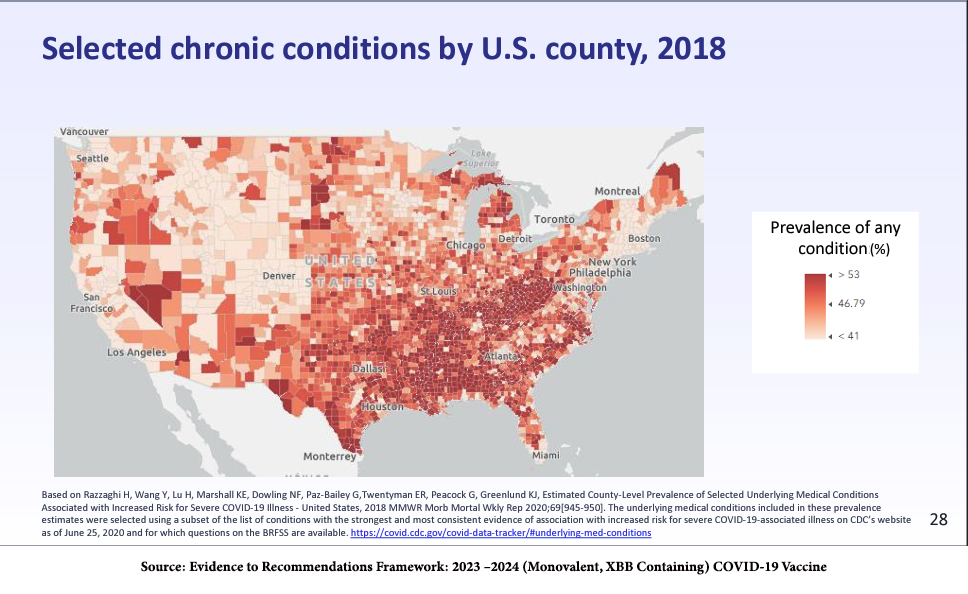
However, the absence of comorbidities does not mean you will avoid serious illness, hospitalization, or death from a COVID-19 infection. As with many aspects of the COVID-19 pandemic, it’s all a matter of probabilities.
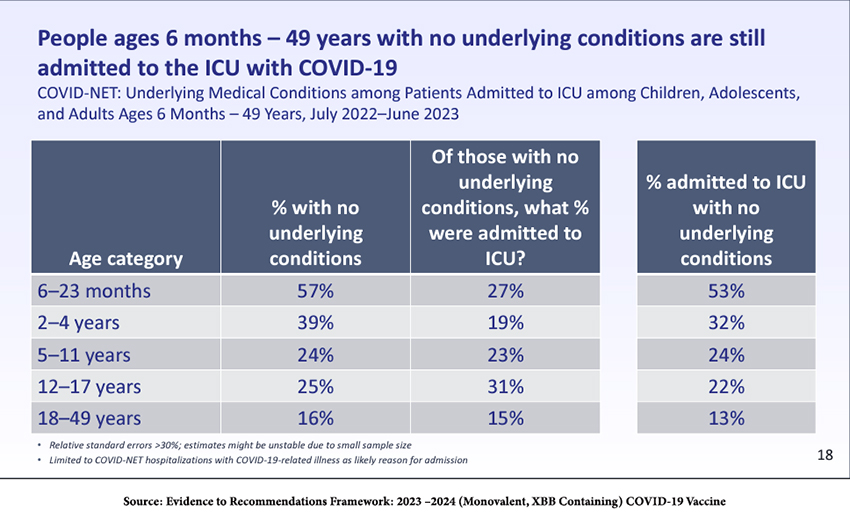
As the table shows, although most young children have no comorbidities, many become seriously ill and are admitted to the ICU.
Moreover, COVID-19 is not influenza; it results in greater hospitalizations than other diseases that can be treated with childhood vaccines and more pediatric deaths than influenza.
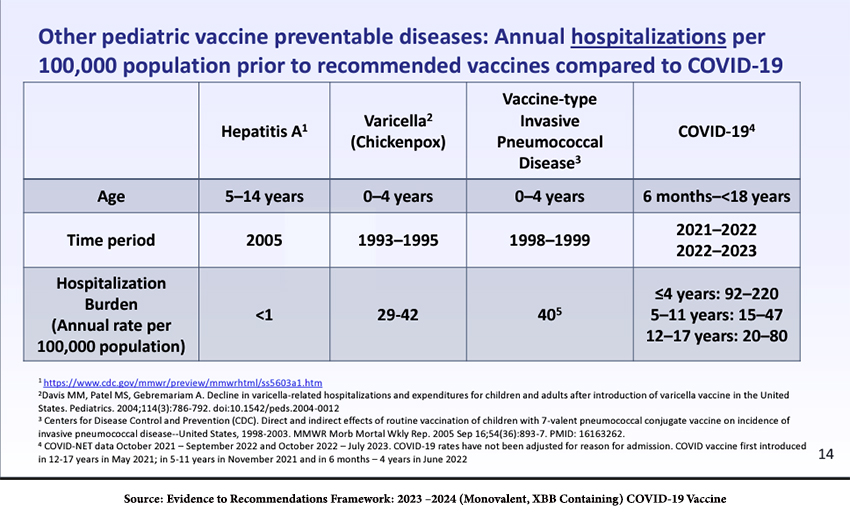
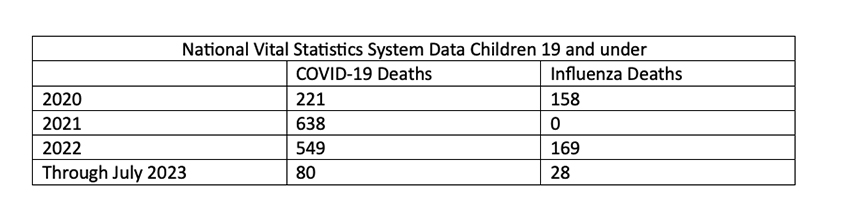
Vaccine Efficacy
If there is anything equivocal about the recommendations of these newer COVID-19 vaccines, it is the absence of clinical data on vaccine efficacy. Unlike the case for the initial vaccines, there are no available randomized, controlled studies, the “gold standard.” There simply isn’t sufficient time or enough COVID-19 infections in the community to show a difference in infection rates between vaccinated and placebo-treated groups.
But that is not unusual in vaccine development. Each year’s new influenza vaccines do not undergo clinical trials for efficacy. Rather, the FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) makes the final decision in the Spring about vaccine viruses for domestic flu vaccines that will be administered in the Fall. Information about the circulation of flu viruses and available vaccine viruses is summarized and presented to VRBPAC in February or March each year to determine which viruses to include in the upcoming season’s flu vaccines. A dozen or more manufacturers of flu vaccines then begin to produce them using a variety of validated platforms or technologies. There are small studies to ascertain that side effects are acceptable and that the vaccines elicit appropriate levels of antibodies and cellular immunity.
The pooled estimates from observational studies in adults and adolescents after last year’s bivalent boosters showed about 48% efficacy in preventing hospitalization and 61% efficacy in preventing death.
"VE [vaccine effectiveness] findings should be interpreted as the incremental benefit provided by COVID-19 vaccination in a population with a high prevalence of infection-induced immunity."
- Ruth Link-Gelles, PhD, MPH, team leader for the COVID vaccine effectiveness program at the National Center for Immunization and Respiratory Diseases (NCIRD)
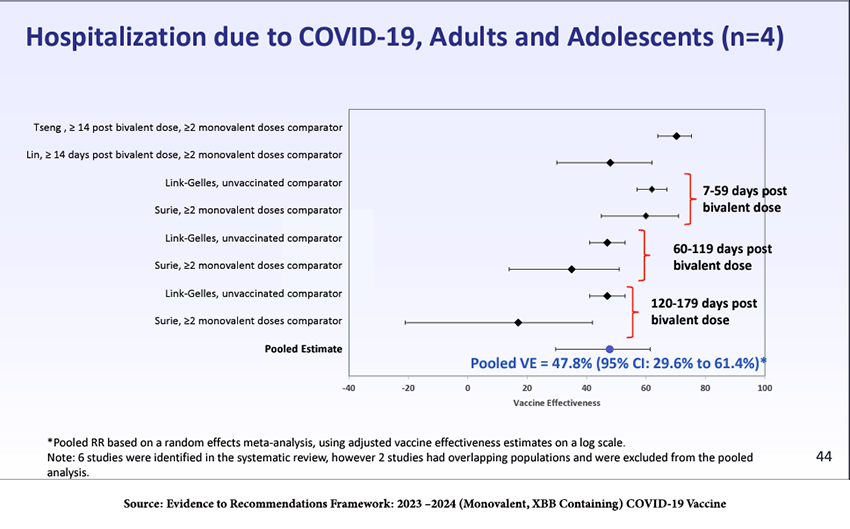
Thus, although the incremental immunity afforded to adults and adolescents from last year’s bivalent vaccine was substantial (top line of the figure), it waned significantly by 179 days post-administration. There is insufficient data on visits to the ED, hospitalizations, or deaths in children to draw conclusions. Data on adolescents and children were used to “model” their responses.
Vaccine Safety
Let’s begin with two of the most widely discussed concerns: myocarditis and pericarditis.
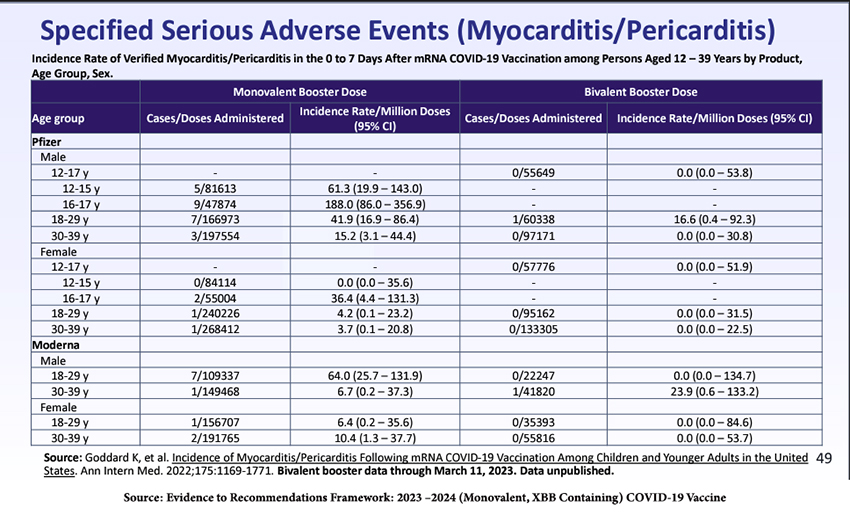
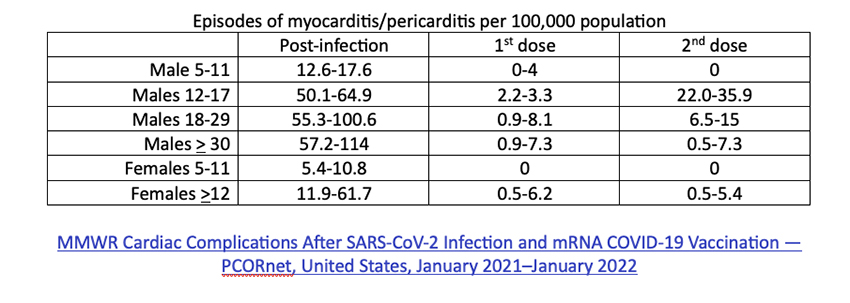
There is an increase in post-vaccination myocarditis and pericarditis, especially in males 16-17, and more from the Pfizer product than Moderna’s. Males aged 30-39 seemed more affected by the Moderna bivalent booster. We have learned that the incidence of myocarditis can be reduced by longer intervals between doses and by individualizing which of the mRNA vaccines is given.
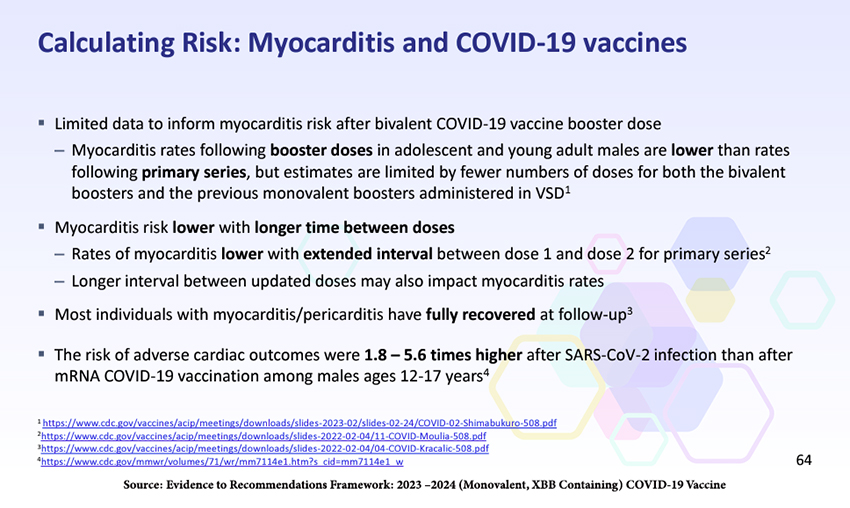
The last of those points is especially pertinent because it puts into perspective the disinformation about the supposed myocarditis risks of COVID-19 vaccines promulgated by the anti-vaccine fearmongers.
A more in-depth look at the recovery of myocarditis patients found that at 90 days, “most patients reported no impact on the quality of life, and most did not report missing school or work.” A small percentage, 13%, were readmitted to the hospital, but there were no “vaccine-associated myocarditis deaths in this group.”
Dr. Marty Makary, one of the most persistent and vocal anti-vaccine COVID-minimizers, wrote in an opinion piece in the New York Post:
"…for young people, the incidence of myocarditis is six to 28 times higher after the vaccine than after infection, even for females, according to a 2022 JAMA Cardiology study."
But, a careful look at the study’s methodology indicates that the post-vaccination incidence of myocarditis was compared to the incidence of myocarditis in 2017-2019, long before COVID-19 was identified and certainly before the pandemic. Thus, the comparison could not have been to COVID-19 infection. Moreover, the article states that it is a “[d]escriptive study of reports of myocarditis to the Vaccine Adverse Event Reporting System (VAERS) that occurred after mRNA-based COVID-19 vaccine administration,” but as spelled out clearly on the CDC website:
“Specifically, a report to VAERS does not mean that a vaccine caused an adverse event” [emphasis in the original]
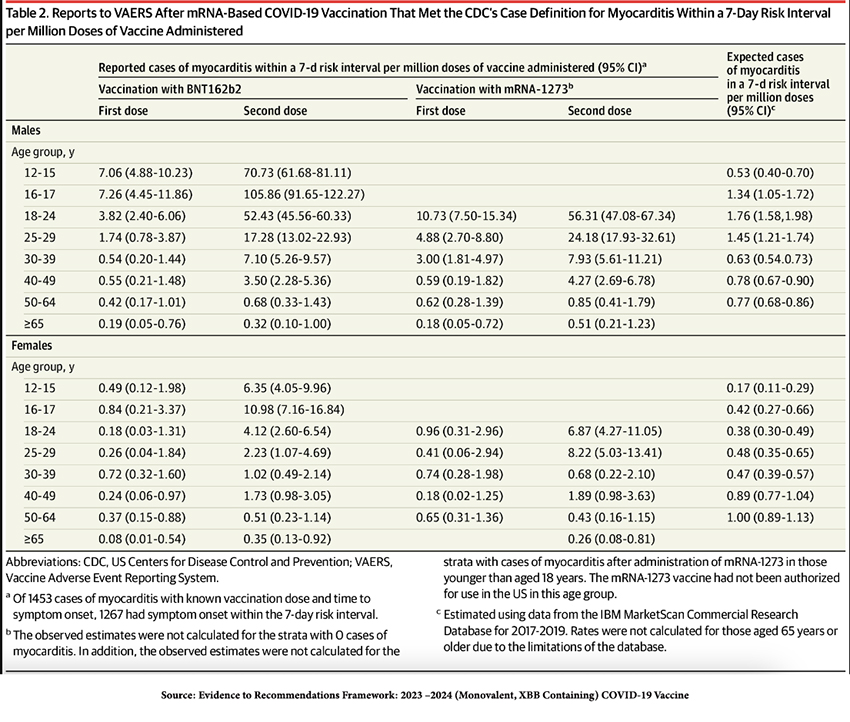
Finally, in every age group, the incidence of myocarditis and pericarditis vaccines is far higher post-COVID-19 infection than post-vaccination.
Those findings directly contradict Makary’s assertions.
Dr. Makary also wrote that there was
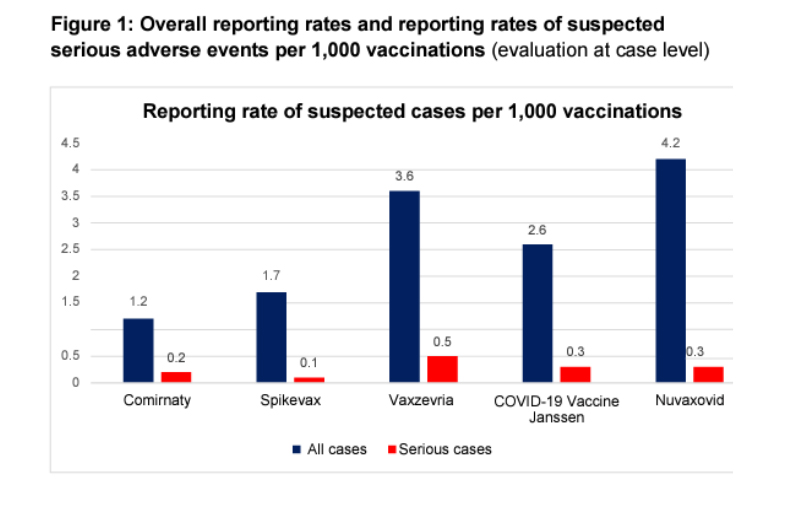
“a serious adverse event rate of one in 5,000 doses, according to a German study by the Paul-Ehrlich-Institut.”
As the graphic below from the study shows, these were suspected rather than confirmed cases. (Additionally, would you consider fatigue and headache severe?) Finally, note that those suspected adverse events were significantly lower in Comirnaty and Spikevax, the two COVID-19 vaccines currently available in the US—more misdirection from Dr. Makary.
The Public’s Attitude
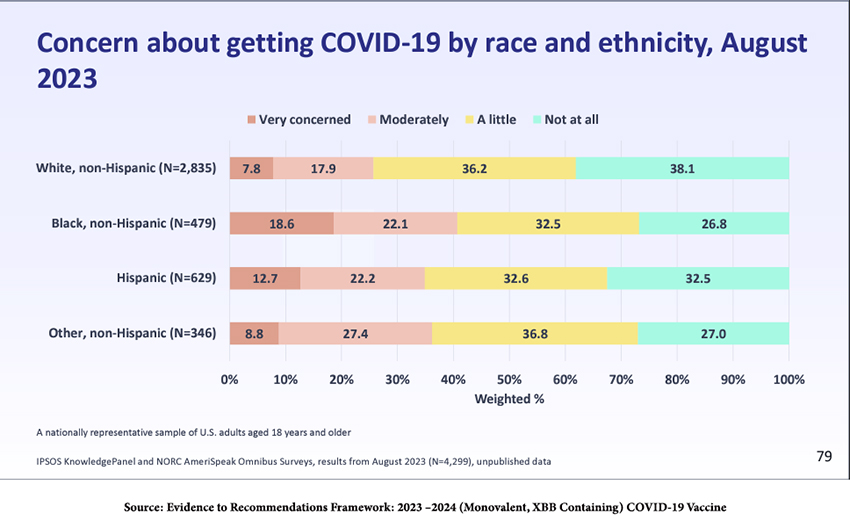
Understanding the public’s view of COVID-19 helps to frame what will and will not be acceptable public health measures. As illustrated by the poor rate of uptake of the last round of boosters – around 17% of the US population – and the near-disappearance of masks even in high-risk situations, many people seem to believe that the danger of COVID-19 has passed. The results of the survey below reflect that. In the aggregate, roughly 30% of the US is worried.
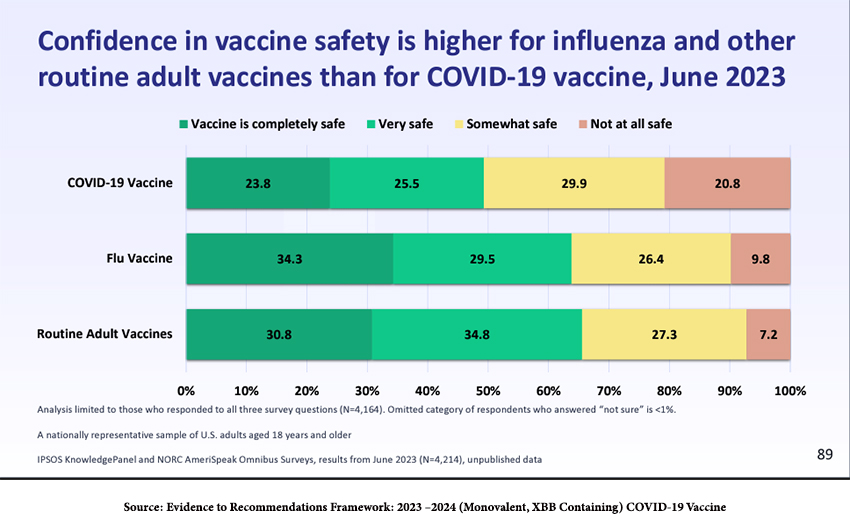
How can we expect the new round of vaccines, now becoming available, to be received? Confidence in COVID-19 vaccines is lower than for other routine adult vaccines, which is not reassuring.
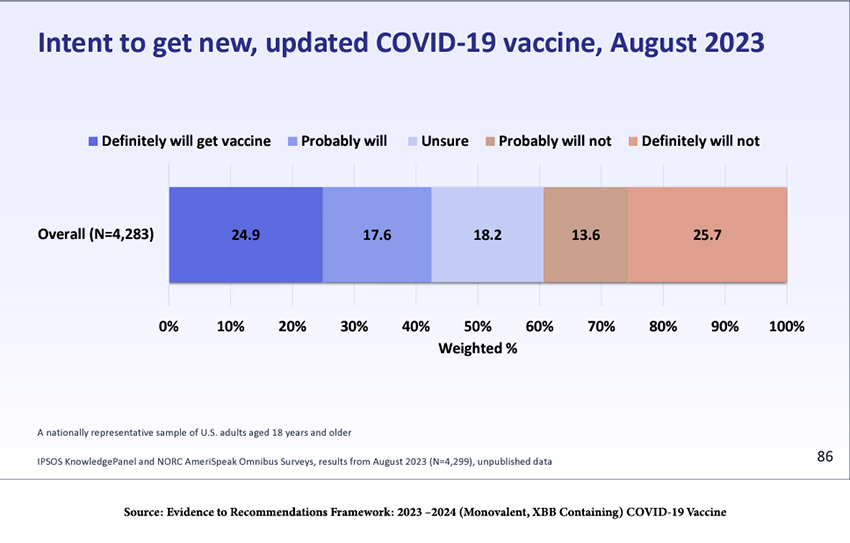
According to an August survey (see the figure below), roughly a quarter of the population will get the vaccine, while another quarter “definitely will not,” and the remainder are undecided. That bodes ill, especially if new, more transmissible SARS-CoV-2 variants become dominant.
 Prior behavior is probably the best predictor of peoples’ attitudes toward a new vaccination. As noted in the presentation to ACIP, twenty-eight percent of those individuals who had at least one vaccine dose previously will probably not get the new vaccine, and that number rises to 88% among those who have never been vaccinated. Public health education and recommendations should probably be directed to the potentially convincible middle 35%.
Prior behavior is probably the best predictor of peoples’ attitudes toward a new vaccination. As noted in the presentation to ACIP, twenty-eight percent of those individuals who had at least one vaccine dose previously will probably not get the new vaccine, and that number rises to 88% among those who have never been vaccinated. Public health education and recommendations should probably be directed to the potentially convincible middle 35%.
That educational effort is likely to be an uphill battle if even the medical community is not fully onboard:
“A June 2023 survey of just over 4,000 adults showed that confidence in vaccine safety is higher for influenza and other routine adult vaccines than for COVID shots. Data from that same survey showed that healthcare providers had far lower rates of recommending COVID shots compared with flu or other routine vaccines: 56.2% for COVID versus just above 60% for shingles and pneumonia, and about 72% for flu.” [emphasis added]
Overall
- Vaccination is highly cost-effective in persons aged>65 and, to a lesser degree, those >50.
- Vaccination for people aged 18-49 was “sensitive to changes in input,” meaning that the benefit more readily outweighed the harm as an individual’s risk (from comorbidities) rose or if the vaccine had greater efficacy against hospitalization than modeled.
- A clear benefit-harm analysis could not be made for the general pediatric population.
An often-ignored and important factor is that vaccination reduces the likelihood of post-COVID-19 persistence or appearance of new signs and symptoms – that is, Long COVID), which represents not only potential severe disability of individuals but long-term stress on the nation’s healthcare infrastructure. If the estimate below from the CDC’s National Center for Immunization & Respiratory Diseases is correct, almost 15% of post-COVID-19 infection patients in the US are currently suffering from Long COVID, a staggering number.
What to do?
The ACIP panel, which was charged with making public health recommendations, considered many factors:
- Benefits could be anticipated for all age groups, but the degree of benefit varied with age.
- The lowest burden of disease was among children ages 5-17, but deaths in this age group were reported, and half of the children had no underlying risk factors.
- For the males at risk for myocarditis, the risk seems to be lessening as we extend the interval between doses.
- The “vast majority of the US population has an underlying condition that would qualify under a risk-based recommendation.”
- Modeling projected a more significant reduction in hospitalization and deaths when vaccination was universally employed compared to making no recommendation or targeting only those individuals >65.
- A universal recommendation may increase vaccine uptake as it is easily remembered and less subject to exceptions that change over time.
“Antiscientism feeds on the disillusionment over science’s inability to deliver definitive visions of the world—on the fear of accepting ignorance. False certainties are preferred to lack of certainty.”
- Anaximander by theoretical physicist and philosopher Carlo Rovelli
Unfortunately, there is a lot of antiscientism about. Physicians like Makary and Florida Surgeon General Joseph Ladapo relentlessly misinform, mislead, and endanger the public. We have presented the information that informed the CDC’s decision to recommend the most current iteration of COVID vaccines for nearly all of the general public. We hope this will convince most Americans to opt to take the new COVID-19 vaccines. That would benefit the vaccinated themselves, their families, and the community.
Source: Evidence to Recommendations Framework: 2023 –2024 (Monovalent, XBB Containing) COVID-19 Vaccine
The real data behind the new COVID vaccines the White House is pushing New York Post
Update: Epidemiologic Characteristics of Long COVID Report from National Center for Immunization & Respiratory Diseases September 12, 2023