When biologists think of evolution, we tend to be biased toward those instances in which an organism gains some new ability, such as when a bacterium acquires a new antibiotic resistance gene. In other cases, new traits can be conferred by gene duplication, in which an extra (and usually nonessential) copy of a gene mutates and acquires new functions. Traditionally, but incorrectly, an assumption exists that more complex organisms have larger genomes. As a result, biologists have tended to view evolution as a process by which organisms amass new genes and functions over time.
But in a new paper published in Nature Reviews Genetics, University of Barcelona geneticists Ricard Albalat and Cristian Cañestro warn that this is far too narrow a view. They contend that gene loss, and the loss-of-function that generally accompanies it, may play a large and overlooked role in evolution.
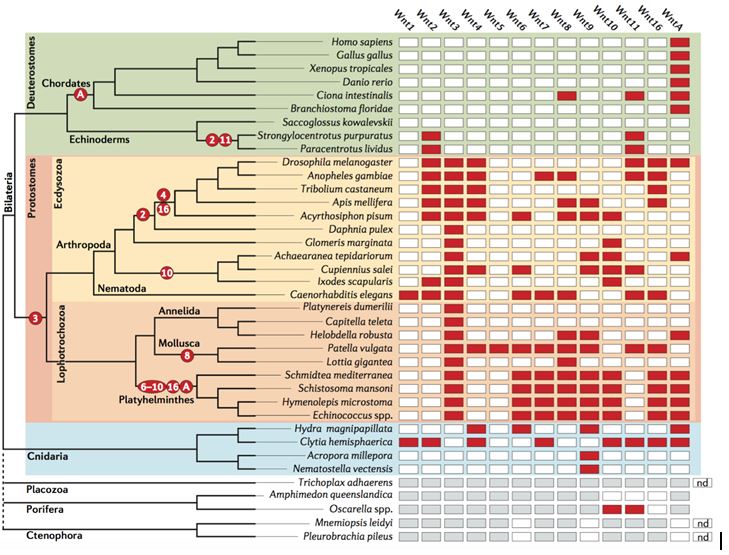
The authors focus much of their discussion on the Wnt family, a large group of genes present in all animals that encode proteins important for cellular signaling. During the course of animal evolution, some of the Wnt family genes were lost. (See figure. Red boxes indicate gene loss.)
There are two things worth nothing from the chart. First, the rate of gene loss is different for different animal phyla. Notice, for instance, that the fruit fly (Drosophila melanogaster) and a roundworm (Caenorhabditis elegans) have lost many more Wnt genes than humans. Second, certain gene loss events may have been crucial to the evolution of entire lifeforms. The loss of WntA preceded the evolution of chordates (animals with spinal cords), and as a result, no higher animals possess WntA.
Paradoxically, experiments have shown that most genes appear to be nonessential. If given a hearty food supply, some bacteria can do without 90% of their genes. A similar result was shown using human cancer cells grown in the laboratory. What explains this? The authors offer two hypotheses.
First, it is possible that there are “backup” genes performing similar functions or alternative metabolic pathways available to the organism. Evidence for this hypothesis comes from experiments that show the percentage of supposedly “nonessential” genes falls dramatically when multiple genes are deactivated.
Second, a gene may be nonessential only under certain conditions. Consider the metabolic pathway that synthesizes vitamin C. Humans and several other animals lack a key gene in this pathway. Because we have a large and abundant supply of vitamin C in our diet, this gene is no longer essential. However, if something were to ever happen to the food supply and vitamin C went missing, we would be in real trouble. (This is also an example of regressive evolution. Since our primate ancestors probably ate a lot of fruits and vegetables, the genes for vitamin C synthesis were no longer necessary.)
Ironically, gene loss itself can be adaptive and confer new traits. Dropping unnecessary genes appears to be important for the success of parasites. In humans, the ABO blood group is determined by an enzyme that attaches a sugar to blood cells. People with type O blood have a nonfunctional enzyme, but they also appear to be more resistant to malaria.
Thus, biologists ought to keep in mind that what an organism loses may be just as important as what it gains. From an evolutionary perspective, sometimes less really is more.
Source: Ricard Albalat & Cristian Cañestro. “Evolution by gene loss.” Nature Reviews Genetics 17: 379-391. Published online 18-April-2016. doi:10.1038/nrg.2016.39