When it comes to finding new antibiotics, no place is too weird to look.
Last week, we reported that two species of fungi, both isolated from an acidic, metal-rich lake, cooperate to synthesize an antibiotic that neither produces when grown alone. Now, three separate teams of researchers have identified potentially useful antibiotics from some of the strangest places imaginable: Sponges, sea snails, and marine worms. All reports were published in the Journal of Natural Products.
In the first study, a team of mostly Japanese scientists isolated a compound called Zamamidine D from a sponge named Amphimedon. The compound displayed strong antifungal activity against Cryptococcus neoformans (which infects immunocompromised patients) and moderate antibacterial activity against Staphylococcus aureus and a few other bacteria. (Last year, we reported on an Antarctic sponge that produced an antibiotic called darwinolide, which was active against MRSA.)
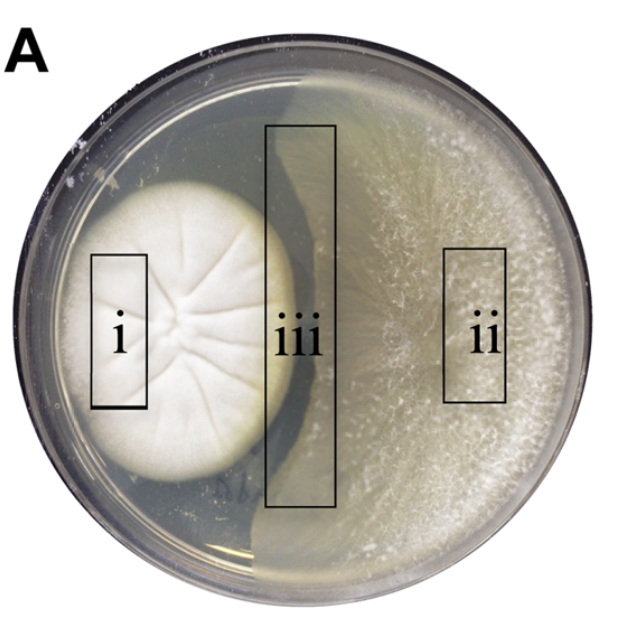 In the second study, Australian scientists isolated two fungal species from a sea snail, Siphonaria. When grown in co-culture, one fungus produced a compound that appeared to inhibit the growth of the other fungus. (See picture, region iii.) When grown by itself, the fungus did not produce the presumed antifungal agent, leading the researchers to speculate that these fungi were engaged in a battle that triggered chemical warfare.
In the second study, Australian scientists isolated two fungal species from a sea snail, Siphonaria. When grown in co-culture, one fungus produced a compound that appeared to inhibit the growth of the other fungus. (See picture, region iii.) When grown by itself, the fungus did not produce the presumed antifungal agent, leading the researchers to speculate that these fungi were engaged in a battle that triggered chemical warfare.
Finally, in the third study, a team of Chinese researchers isolated six new compounds from a species of fungus called Penicillium (the same genus that produces penicillin) living inside of a marine worm, Sipunculus nudus. One of the compounds inhibited the growth of Staphylococcus aureus, albeit weakly, while three other molecules demonstrated an ability to block the growth of H1N1 influenza.
As it turns out, the ocean is a great place to hunt for new antibiotics. A review, also published in the Journal of Natural Products, reported that from 2010 to 2015, more than 50 different antibiotics had been isolated from marine bacteria.
Limitations
Many people worry that isolating new antibiotics will do nothing except make antibiotic resistance even worse. That's probably true, but there are few, if any, alternatives. Evolution is an arms race. Bacteria are genetically flexible and quite capable of adapting to radically new environmental conditions. The trick, therefore, is not to give up but to outsmart them with better antibiotic management and innovative therapies.
All of these studies face the same limitations. Years of work remain before these basic discoveries can be translated, if ever, into a medical setting. It is quite possible that these antibiotics, for various reasons, will prove commercially unviable or clinically useless. On the other hand, novel compounds that are not antimicrobial can be modified by chemists to produce semi-synthetic compounds that are.
So, the search continues.
Sources
(1) Takaaki Kubota, et al. "Zamamidine D, a Manzamine Alkaloid from an Okinawan Amphimedon sp. Marine Sponge." J Nat Prod 80(4): 1196-1199. Published: 16-Feb-2017. DOI: 10.1021/acs.jnatprod.6b01110
(2) Zhuo Shang. "Chaunopyran A: Co-Cultivation of Marine Mollusk-Derived Fungi Activates a Rare Class of 2-Alkenyl-Tetrahydropyran." J Nat Prod 80(4): 1167-1172. Published: 6-Apr-2017. DOI: 10.1021/acs.jnatprod.7b00144
(3) Fan-Dong Kong, et al. "Chrodrimanins K–N and Related Meroterpenoids from the Fungus Penicillium sp. SCS-KFD09 Isolated from a Marine Worm, Sipunculus nudus." J Nat Prod 80(4): 1039-1047. Published: 17-Feb-2017. DOI: 10.1021/acs.jnatprod.6b01061




