No matter how safe DDT may be (and it's probably far safer than you think), there can be no downside to using less of it, provided that it works as well. Thanks to chemists at NYU, this may become possible. Pretty cool. But before we go into this...

The obligatory chemistry lesson! Don't skip it. I'll find out. (BTW, just for yuks. Anyone know what the music is? The notes are real.)
Obligatory Chemistry Lesson:
One of the many headaches that drug discovery chemists face is a preternaturally annoying problem called crystal polymorphism. It can kill an otherwise promising drug unless it can be fixed, and sometimes it can't.
Crystallization (1) is almost always the best way to purify an organic compound (2). The process of having a chemical compound form crystals from a hot supersaturated solution excludes impurities that may be present. Only the pure compound will form the crystal, and an amazingly pure material is obtained in this manner. That is when it is behaving right. It doesn't always.
Depending on the compound in question and the solvent from which it crystallizes, sometimes polymorphs can form. Polymorphs are different shaped crystals, often both of equal chemical purity. But chemical purity is not sufficient for the bulk drug (the final material that is put into capsules or made into tablets. This is because different crystal forms, even though they may both be 100% pure, will have different properties, especially melting point and solubility, both of which affect absorption of the drug in the gut. And when your pure solid is a mixture of polymorphs, the composition of any two batches will be different. This is a big no-no for drugs.
Here is how this situation typically plays out in a lab of a drug company:
- Layoff rumors abound.
- Management tells chemist that the big mess that he/she is handed must be made 99.99% pure in two days.
- Management, which couldn't crystallize salt in a freezer, does not realize (or care) that this is impossible. They just want their bonuses.
- After four months and hundreds of different crystallization tricks, the chemist has 99.99% pure material.
- Chemist sends the crystals to an analytical chemist who examines the crystals under a microscope.
- Analytical chemist reports polymorphism.
- Original chemist hangs himself.
- The chemist is not replaced because the layoff rumors were true.
- Management gets bonuses.
Back to the polymorphs. You can just about hear this poor guy screaming:
"Selected and scaled up "Form C" in pilot plant where "Form D" was discovered and began to predominate. Aiieeeee!"
Mahavir Prashad, Ph.D.
Novartis' Mahavir Prashad, Ph.D. not only had to endure the misery of four polymorphs (Figure 1) but also got into this article. Not sure which is worse:


Figure 1 - An example of different crystal forms of the same compound. Source: Mahavir Prashad, Org. Process Res. Dev., 2010, 14 (4), pp 878–882
As bad as polymorphism may be for chemists, it could be even worse for mosquitos, thanks to some very clever observations (and also luck) by Professor Bart Kahr and colleagues of the NYU chemistry department.
Kahr's group, which studies the properties of crystals, and was working with DDT which because of its chemical symmetry, forms a different type of crystal which was of interest to the group. Then they got lucky (and also smart). The group noticed that DDT came in (at least) two polymorphic forms, no matter how it was synthesized. They called the two forms Form 1 and Form 2. Perhaps not the most creative nomenclature ever, but let's cut them a break.

Figure 2: Two DDT polymorphs. Source: "DDT Polymorphism and the Lethality of Crystal Forms." Angew. Chem. Int. Ed. 2017, 56, 1 – 6.
This is where things get interesting. Mike Ward, who collaborated with Kahr explains:
‘Each polymorph expresses inherently different crystal structures and faces. On each face the molecules are oriented differently and this means that the interactions between the molecules with the footpads of insects are different.’
Professor Mike Ward. NYU chemistry department.
This means that the lethality of DDT depends on both its chemical and crystal structure. Form II was significantly more lethal to fruit flies, possibly because its crystal form provides a greater exposed surface area. Let's take a closer look:

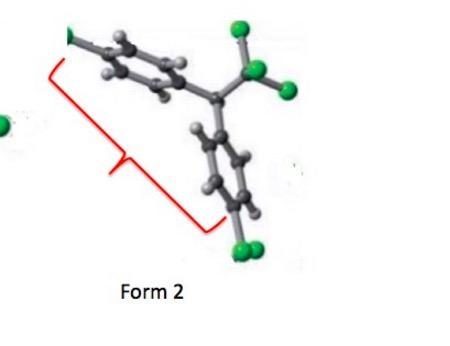
An enlarged, simplified version of Figure 2. The two benzene rings are in gray. The green spheres represent the chlorine atoms. In Form 1 (left) one of the benzene rings is turned so that you are seeing it edge on.
I removed everything from the above figure except for one crystal unit of each form, which makes it much easier to see the differences in exposed surface areas of the two forms. Note that in Form 1 the two rings are folded such that they are close to each other, while in Form 2, they are "stretched out," (red bracket) which results in the greater surface area for this polymorph.
So, why not just make DDT in Form 2 and use less of it, and make everyone (more or less) happy. It's the same reason that poor Mahavir Prashad was pulling his hair out—polymorphs. Right now it is impossible to make pure Form 2. But Professor Kahr thinks they can overcome this (3):
"I don’t think that it should be an insurmountable problem... [W]e think that we can probably achieve this with more work."
Maybe, maybe not. These guys may be able to pull it off, but it's no easy task. And please, don't give this job to Mahavir Prashad. He has suffered enough.
Notes:
(1) A more accurate term is 'recrystallization,' since most impure solids are already (at least partly) crystalline. It is something organic chemists do thousands of times in their careers. The crude solid is dissolved in the correct volume of a boiling solvent and allowed to sit while the purified crystals form. Sometimes this must be repeated numerous times. What solvent to choose? How much? This is the art of crystallization.
(2) I hate to brag, but I wasn't named "Billy Crystal" in my lab for no reasons. OK, that's a lie. I gave myself the name. But when people of lesser abilities could not get something to crystallize, they visited my lab, at which point I usually had it done in about a minute. Arrogant? Perhaps.
(3) For a very interesting look at polymorphism, the incomparable Chemjobber (I know the guy. He has to do stuff like this for a living) see his blog piece "Puzzling Polymorphs."



