The utility of mammography screening for breast cancer has been a bone of contention, but for some women it has been a life-saver. Indeed, the US Preventive Services Task Force currently recommends that women ages 50-74 should get a mammogram every 2 years. These recommendations are for women who have not been previously diagnosed with the disease, who have no family history or known mutation (e.g BRCA1 or 2) that increases their risk.
The CDC recently reported that the rate for screening tests for breast cancer was below the Healthy People 2020 goal of 81 percent — between 2000 and 2015 it was stable at about 72 percent. While the likelihood that women would get a mammogram was higher for women with more education and higher incomes, another factor might be involved in a woman's avoidance of the procedure — it's unpleasant and can be downright painful.
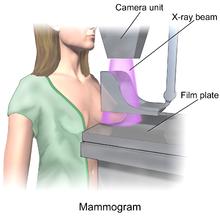
A mammogram is basically a low-dose x-ray picture of the breast, and the picture is "read" by a radiologist who can spot abnormalities, and also may compare the current picture to past ones of the same person. The procedure involves briefly flattening the breast between plastic plates while the x-ray is taken — usually just a few seconds. The degree of compression varies with the size and density of the breast tissue in order to obtain the clearest image possible. While this is basically a benign procedure, it is not necessarily pleasant as the degree of compression, which is administered by the technologist, can be painful.
One way to make mammography more bearable for more women might be to give them more control of the procedure. And the FDA recently approved a device to do just that. Their description of the procedure reads:
The system has a handheld wireless remote control that patients can use to adjust the compression force after breast positioning. During a mammography exam, the technologist positions the patient and initiates compression. The technologist then guides the patient to gradually increase compression using the remote control until adequate compression is reached. The technologist checks the applied compression and breast positioning and makes the final decision on whether the compression is adequate or needs to be adjusted.
In the approval statement, the FDA noted that use of the device would not have a negative impact on image quality, nor did it increase the time needed for the exam. While use of the remote control won't change the necessary degree of compression needed for a clear image, it might be more acceptable to women if they feel that they have more control over the procedure. Only time will tell if this will help move the rate of mammographic screening closer to the Healthy People 2020 goals.