Based on the recent demise of experimental vaccines from Genocea and Vical the prospects for a therapeutic vaccine for genital herpes look mighty bleak at this time. So, for most patients who need treatment valacyclovir (Valtrex) is the only choice (1,2). It works well for most people, but not for everyone.
DRUGS VS. VACCINES FOR TREATMENT OF INFECTIOUS DISEASES
There has never been a therapeutic vaccine for any infectious disease, and there isn't one on the horizon. But there are plenty of drugs that work quite well for infections - antibiotics, antifungals, and antivirals. Since herpes is a viral infection is it not unrealistic to predict that an antiviral drug that works better than Valtrex could be discovered. Based on the stunning success of antiviral drugs for treating HIV (3) and hepatitis C (4), most of them coming out of the pharmaceutical industry, it is reasonable to think that this is not only possible but maybe even likely that alternative drugs could be discovered. And one already has been. There is an alternative to Valtrex in Phase II trials right now. The drug is called pritelivir, and it is looking quite good in clinical trials. More on that later.
SOME DRUGS INHIBIT SYNTHESIS OF VIRAL DNA AND STOP REPLICATION
Valtrex/acyclovir (5) work because they inhibit an enzyme called herpesvirus DNA polymerase, which is responsible for the assembly of new viral DNA, a necessary step in the virus's replication. The polymerase builds new DNA by precisely combining 150,000 individual nucleotides - the pieces that make up all genetic material (Figure 1).

Figure 1. Representation of how DNA polymerase works. DNA consists of a very long chain of covalently bound deoxyribose (a sugar, orange circle) units. The polymerase has one function - to hook more sugars together (yellow arrow). This is how the chain grows. The sugar is also bound to one of four nitrogenous bases, A, T, C, G (red arrows). The bases hold serve to connect (via hydrogen bonding) one single strand to another to form double-stranded DNA. The newly formed bond is shown by the green line. Source: YouTube
THE HIV INHIBITOR STRATEGY APPLIED TO HERPES
If you recall the early days of AIDS, the first approved drug (1986), AZT, inhibited an enzyme called reverse transcriptase, which, like acyclovir, is responsible for assembling new viral DNA. So, although the two drugs are acting on entirely different viruses, both acyclovir and AZT inhibit the same essential step in viral replication - building new genetic material so that the virus can replicate.
But AZT, the first drug approved for AIDS (1986), was useless in HIV-infected patients because the virus mutated very quickly to a strain which was resistant to the drug. It was not until 1995 when Roche's Invirase was approved that there finally was a weapon against the virus - the cocktail. By combining two or more drugs that work by different mechanisms resistance is minimized. It is much more difficult for the virus to mutate in two locations at once.
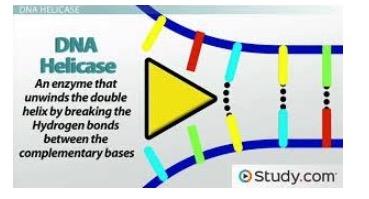
There are now eight classes of AIDS drugs (6), each working by inhibiting a specific step that is critical for replication. All therapies consist of 2-4 different drugs from different classes. But there is one step in HIV DNA synthesis pathway for which there are no drugs - separating the incoming double-stranded viral DNA, which is a necessary step for the virus to make more DNA copies. This is done by an enzyme called helicase. Although no one has figured out how to inhibit helicase in HIV it is precisely this enzyme that is shut down by pritelivir in HSV (Figure 2).

Figure 2. A cartoon illustrating how helicase (yellow arrow) breaks the hydrogen bonds that hold the two strands of double-stranded DNA together producing the single-stranded DNA that is required for viral DNA synthesis. Source: Study.com
HIV AND HSV STRATEGIES DIVERGE - MORE OR LESS
Just because there is no helicase inhibitor drug for HIV does not mean that the enzyme is not a valid target in other viruses. Pritelivir works by preventing the helicase enzyme from breaking the hydrogen bonds that hold the strands together. Double-stranded DNA cannot be used to make more DNA; only the single strands can do this.
IF YOU HAVE MADE IT THIS FAR WITHOUT HANGING YOURSELF: HOW WELL DOES IT WORK? PRETTY IMPRESSIVE
A drug that is different but no more effective than Valtrex would be of some use, but one that outperforms it would be a significant advance. According to an article in Contagion Live, pritelivir did this rather well in a 28-day double-blind Phase II trial. The 91 participants had suffered 4-9 outbreaks in the past year. The results (Table 1) are impressive. Pritelivir works about twice as well as Valtrex.

Table 1. Comparison of Valtrex and pritelivir in genital herpes patients.
WHAT'S NEXT?
The FDA has granted pritelivir fast-track status, (7) which is reserved for promising drugs that treat serious conditions or unmet medical needs, and moves the drug to the front of the line. The benefits of fast-track status include:
- More frequent FDA meetings to examine the development plan and suggest additional data that are needed.
- More frequent written communication between the company and the FDA
- The possibility of getting Accelerated Approval and Priority Review, which means that the FDA will reach a decision within six months, usually a one year process.
- The company can submit sections of the New Drug Application for review rather than the entire document - a process termed a rolling review.
PREDICTION
Given the need for better herpes drugs, the efficacy of the drug in small trials, and the absence of serious side effects, pritelivir ought to have smooth sailing to Phase III.
OUT ON A LIMB PREDICTION
Pritelivir will be approved, possibly within two years. Yes, I know I'm out of my mind to predict this.
OUT ON A LIMB HOLDING AN ANVIL PREDICTION
Based on the AIDS model, pritelivir will be tested in combination with Valtrex and the results will be additive or maybe even synergistic. This would be incredibly good new for those who have been constantly disappointed by the vaccine failures. Despite the absence of a vaccine, drug therapy will be improved enough to significantly reduce outbreaks, shedding, and transmission. A combination therapy COULD be life-changing for the millions of Americans who suffer from this nasty infection.
I sure hope I'm right this time. Scientifically my predictions are reasonable, but there is still plenty the could go wrong as pritelivir makes its way through the approval process.
Keep those fingers crossed.
NOTES:
(1) There are other drugs available. Valtrex is a pro-drug, which breaks down in the blood to give acyclovir, the active drug. But sometimes doctors prescribe acyclovir instead. I don't get this at all. Valtrex is far better absorbed so that it can be taken 1-2 times per day instead of 4-5 times for acyclovir. Blood levels of acyclovir are higher when Valtrex is used compared to acyclovir itself.
(2) Famvir (famciclovir) is another antiviral pro-drug, which is converted to penciclovir, the active drug. Although Famvir that is usually used for shingles, which results from the herpes varicella-zoster virus, it can also be used to treat herpes. It acts by the same mechanism as Valtrex and the two drugs perform similarly.
(3) See 36 Years Since AIDS Hit - Now The Possibility Of A Cure
(4) See Hepatitis C: Academia And Industry Work Together To Find A Cure
(5) Valtrex is a pro-drug of acyclovir. Valtrex itself is not active against the virus but breaks down in the blood, forming acyclovir - the active drug. For a look at how pro-drugs work see ADHD Sufferers—Pay Attention: Here's How Vyvanse Works.
(6) Sometimes you see six. This is because two drugs that do similar things are put into one class. It's a bit arbitrary. I'm going with eight.
(7) Fast-track status is requested by the company.