Antibiotic resistance is spotty. If you are hospitalized in New York and you acquire a Gram-negative infection in the hospital, there is a reasonable chance it will be caused by a highly resistant pathogen. If you go to a hospital in New Hampshire or Vermont, there is almost no chance for that to happen. ACSH advisor Dr. David Shlaes explains.
This is not the MRSA pandemic, ladies and gentlemen. Let's take a look at the MRSA pandemic. In US hospitals, even today (latest CDC data is from 2014), 46% of S. aureus IV catheter, urinary catheter, and surgical wound infections are MRSA. At one point, in the early 2000s, over 70% of S aureus isolates from emergency rooms in the US were MRSA. Although the resistance levels vary from state to state, the range of MRSA is 33-68%.
Looking at the same CDC data set for carbapenem-resistant Enterobacteriaeceae, for example, we can see that the US average is about 3.5% and ranges from 0-12%. The state with 12% levels of resistance is New York, by the way. As of five years ago, there was, therefore, over a 10-fold difference between the incidence of CRE and MRSA infections that are followed by the CDC.
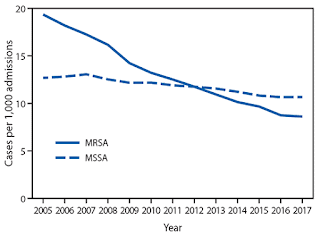
If we now apply these data to US market conditions for antibiotics, the comparisons become enlightening. The MRSA pandemic started in 1968 and accelerated around 1982 or thereabouts. By 2005, over 50% of S.aureus isolates in US hospitals were MRSA. To translate that into actual infection incidence rates, see the figure below from the CDC. In 2005 there were almost 20 MRSA infections per 1000 hospital admissions. Although this number has decreased, as noted above, 46% of strains are still MRSA.
This tremendous medical need drove the market for anti-MRSA drugs for years. Incredibly, the only (mostly) drug available to treat serious MRSA infections was vancomycin. The graph below shows how this market was driven in terms of annual production of vancomycin by Lilly. The market continued to drive the sales of newer agents like linezolid and daptomycin. Whether that will apply to newer anti-MRSA agents is not really clear.

I have been unable to find US case incidence rates for CRE infections. But clearly, this is not the MRSA pandemic. For MRSA infection, we always had vancomycin, and later there was linezolid and daptomycin. Of course, before 2015 and the approval of ceftazidime-avbactam, there was almost no therapy for many of these resistant Gram-negative infections. With emerging resistance to the polymyxin, there was no therapy at all. Now, of course, we have ceftazidime-avibactam, meropenem-vaborbactam, imipenem-cilastatin-relebactam, and plazomicin. With the possibility to combine aztreonam with any of the above, the truly untreatable infections are exceedingly rare today.
Resistance is spotty. If you are hospitalized in New York and you acquire a Gram-negative infection in the hospital, there is a reasonable chance it will be caused by a highly resistant pathogen. If you go to a hospital in New Hampshire or Vermont, there is almost no chance for that to happen. Even within New York, there is considerable variability from hospital to hospital and between geographic areas. My recent survey on the utility of reimbursement rates for expensive new antibiotics active against these resistant Gram-negative pathogens reminded me of the spottiness of this resistance. Those centers that already have a significant resistance problem are already using the expensive drugs with little thought to their expense. They understand that efficacious, non-toxic therapy of serious infections is always cheaper than non-efficacious toxic alternatives. Those centers that simply do not see resistance, or only see such cases very rarely, have not considered adding these new drugs to their formulary simply because they do not need them. In my survey, price seemed to be of little concern. But something must account for the fact that about 35% of CRE infections in the US are still treated with the polymyxins (see this paper by Clancy et al).
The problem is, though, that resistant Gram-negative pathogens are unlikely to go away, and are, in fact, more likely to spread. And, not only that but as Stuart Levy would have pointed out, resistance to the new antibiotics will also crop up. To deal with that, we will need to use the newer drugs we already have and we will need a pipeline of new antibiotics for the future. Not only will we need antibiotics to overcome emerging resistance, but we will need a choice of various classes to provide alternative therapies for drug intolerance and for specific types of resistance. But today, because of the lack of a sufficient level of resistance to drive appropriate use, the market simply does not exist. And without the market, there will be no new drugs.
So – what we need to do is to establish a market in the absence of a medical need that is sufficient to drive the market by itself. We also need to recognize that this is what we are asking. Yes – there is a resistance problem. Yes, there are more and more serious infections that are “difficult to treat.” And yes, there are areas where these infections are common like Greece, Italy, and New York. But all this together, as yet, does not a market make. What we don’t want to do is wait until there are sufficient numbers of highly resistant infections to drive the market. This will be especially true if resistance to the newer drugs emerges – because then it will be at least a 10-15 year wait for new, effective antibiotics.