There are a plethora of drugs and vaccines in the pipeline to treat or prevent COVID-19, the disease caused by the novel coronavirus, SARS-CoV-2. How many of them are likely to be successful?
The best way to answer that question is to examine the success rates of previous drugs and vaccines that have gone through clinical trials. "Success" means that the drug or vaccine eventually received approval from a regulatory body like the FDA.
The conventional wisdom is that most drug and vaccine candidates fail. In this case, the conventional wisdom is absolutely correct. However, the success rate varies wildly depending on the therapeutic area. Oncology drugs are the least likely to succeed, while vaccines are the most likely.
In order to derive the most accurate numbers possible for clinical trial success rates by phase and therapeutic area, a group of authors from MIT analyzed a mountain of data on drugs and vaccines from January 1, 2000 to October 31, 2015. The data set included 406,038 trials (of which 185,994 were unique)1 and well over 21,000 compounds. Their results were published in the journal Biostatistics in 2018.
The key finding from their paper is summarized in the following chart:
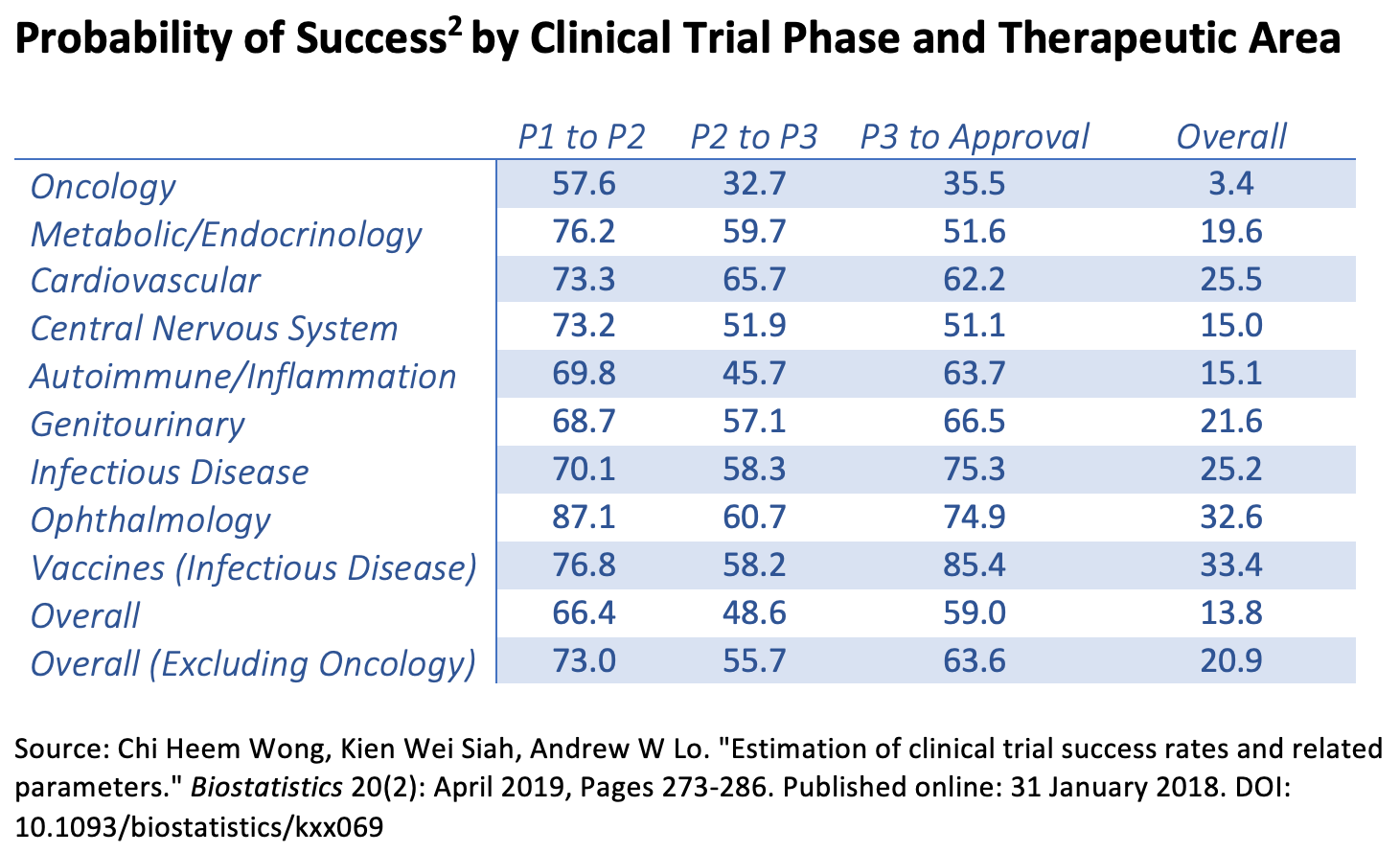
As shown, the overall probability of success for all drugs and vaccines is 13.8%. (If oncology drugs are excluded, the figure is 20.9%.) But this number masks a wide variation by therapeutic area. Oncology drugs have a puny 3.4% success rate, while vaccines for infectious diseases have a 33.4% success rate. That means, for the current coronavirus pandemic, there is an excellent chance that a vaccine will win regulatory approval.
FDA Approval Does Not Mean the Drug or Vaccine Works Well
However, a major caveat is that just because a drug or vaccine is deemed a success by receiving FDA approval does not mean it works particularly well. Why would the FDA approve something like that? Because there aren't any good alternatives. For instance, neither the antiviral drug Tamiflu nor the seasonal flu vaccine are particularly impressive. But, there's nothing better available.
The same concern will remain for any approved coronavirus drug or vaccine. We still don't know if humans develop robust, long-lasting immune responses to coronaviruses. No matter what the FDA says, basic biology ultimately will determine how successful a vaccine is.
Notes
(1) The same drug can go through multiple clinical trials.
(2) Typically, the overall probability of success is calculated by multiplying the probability of success for transitioning from Phase 1 to Phase 2, Phase 2 to Phase 3, and Phase 3 to Approval. However, the methodology used by the authors does not necessarily make that true in this case.
Source: Chi Heem Wong, Kien Wei Siah, Andrew W Lo. "Estimation of clinical trial success rates and related parameters." Biostatistics 20(2): April 2019, Pages 273-286. Published online: 31 January 2018. DOI: 10.1093/biostatistics/kxx069