“The highest burden occurs in countries where health systems are weakest and medical resources sparse.”
Global estimates by the World Health Organization suggest that about half of the 5.4 million snakebites involve venomous snakes, resulting in about 4% fatality and 12% disability or amputation. Thankfully, the US is an outlier. A half-million venomous bites are noted annually in Africa, 2 million in Asia.
“High-quality snake anti-venoms are the most effective treatment to prevent or reverse most of the venomous effects of snake bites.”
Anti-venom is a very effective treatment. It is the historical precursor to monoclonal antibodies being offered as a treatment for COVID-19, and its origin story is reminiscent of that for vaccination. A British officer witnessed and reported in the Madras Medical Journal “a Burmese snake-catcher inoculating himself with cobra venom.” Through the combined efforts of another British army surgeon, a professor of medicine in Edinburgh, and a French scientist, Albert Calmette, working at the then shining star of medical research, the Pasteur Institute, an anti-venom serum was developed.
H.K. Mulford produced the first license anti-venom in the US in Philadelphia in 1927. The company specialized in vaccines both to smallpox and rabies as well as what they termed “antivenin.” His company was subsequently bought by a more prominent firm Sharp and Dohme, which was swallowed to become Merck. Merck continues to produce antibodies for a variety of cancers, e.g., Keytruda and several vaccines, including ones for HPV and pneumonia.
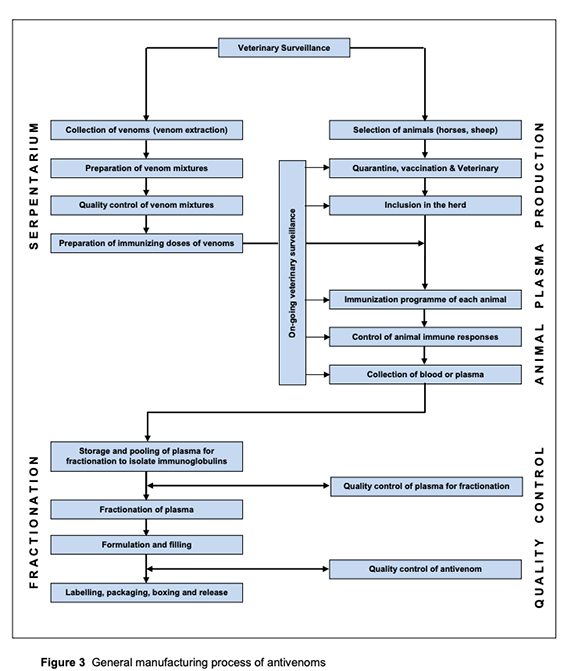 To produce anti-venom, small doses of venom are injected into sheep or horses, and their antibodies, in response to envenomation, are collected, purified, and subsequently injected into a snake-bit victim. We have enough sheep and horses to serve as “bioreactors” to produce anti-venom; the problem lies in distribution.
To produce anti-venom, small doses of venom are injected into sheep or horses, and their antibodies, in response to envenomation, are collected, purified, and subsequently injected into a snake-bit victim. We have enough sheep and horses to serve as “bioreactors” to produce anti-venom; the problem lies in distribution.
Some anti-venoms can be “freeze-dried” to produce a shelf-stable powder to reconstitute when needed. But many of the snake anti-venoms must be stored in liquid form. Much like the Pfizer and Moderna COVID vaccines, these anti-venom requires a cold chain. They require temperatures between 2 and 8 Celsius. Like those mRNA vaccines, getting them into rural areas in low and middle-income countries is quite difficult; unfortunately, that is where they are most necessary.
One more wrinkle to add to the problem is that venomous snakes all produce unique venoms, requiring unique anti-venoms. That is not a complex problem for us; the variety of venomous snakes in the US is limited. But again, in Africa and Asia, where the problem is far greater, many more snake genus cause problems.
Snake bite’s Holy Grail
The ideal anti-venom would treat envenomation by multiple types of snakes. That is possible already by injecting multiple snake venoms into those sheep or horses to produce an antibody response to various venoms or by mixing a variety of single snake anti-venoms together. But again, we run up against the problem of cold chain logistics.
“We think we might have an answer to that: camel antibodies. They have this extraordinary ability to be non-susceptable to heat, so you can store them at room temperature.”
Professor Robert Harrison Liverpool School of Tropical Medicine
His research centers on developing a more stable, more available poly anti-venom, able to treat most snakebites in a particular geography. The heat stability of camel antibodies would eliminate the cold chain hurdle – who knew? Wellcome, the global charitable trust established by pharmaceutical pioneer and entrepreneur Sir Henry Wellcome has put a little over $100 million into the effort. Researchers like Professor Harrison hope that a monoclonal antibody anti-venom will become available in the next five years.
[1] Venomous Snakes National Institute for Occupational Safety and Health
Inspiration for this article came from Why camel antibodies could be the key ingredient in a universal snakebite anti-venom The Telegraph
Additional source material: WHO Guidelines for the Production, Control, and Regulation of Snake Antivenom Immunoglobulins




