
Dr. Phillip B. Messersmith and colleagues from the Department of Bioengineering, University of California, Berkeley, just published a fascinating paper titled "Recyclable surgical, consumer, and industrial adhesives of poly(α-lipoic acid)." Although this title doesn't scream "user-friendly" or "big deal," it has the potential to significantly improve the materials used to close surgical wounds.
Although technically incorrect, let's call "adhesives of poly(α-lipoic acid)" "chemical sutures" for this discussion. And, although the word polymer appears nowhere in the title, this is precisely why this new method works.
Did I hear the word "chemical?" Hmm. You know what this means, right? Another Dreaded Chemistry Lesson from Hell, like it or not.

Steve (left) and Irving, our long-time misanthropic hosts of the TDCLFH®, ponder whether their efforts are sufficiently appreciated. As usual, they have doubts.
What Are Polymers
Polymers are very large molecules composed of repeating units of one or more chemical compounds. The best known is probably Nylon, which was first made in 1935 at DuPont. It's a good example to show one way that polymers form. Here's a simple example of the chemistry responsible for Nylon, and parenthetically, all of life, since these same chemical reactions – the reaction between a carboxylic acid and amine is exactly what forms the polymers of amide bonds (from amino acids) called proteins. (Figure 1).
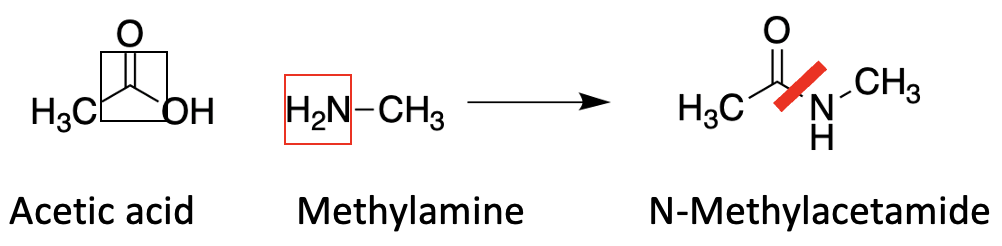
Figure 1. Amide formation occurs when carboxylic acids react with amines. The carbonyl group, represented by a black box, bonds with the amine nitrogen, shown in a red box, to form an amide bond (red line)
Nylon
What does this have to do with Nylon? Please bear with me.
Most polymers can be broken down into two classes: those with a single repeating unit of the same monomeric precursor, X
-X-X-X-X-X-X-X-X-
Or those with repeating units of two different monomers, X and Y
-X-Y-X-Y-X-Y-X-Y-X-
Nylon (1) is an example of the second class, formed from a 6-carbon dicarboxylic acid and a 6-carbon diamine:
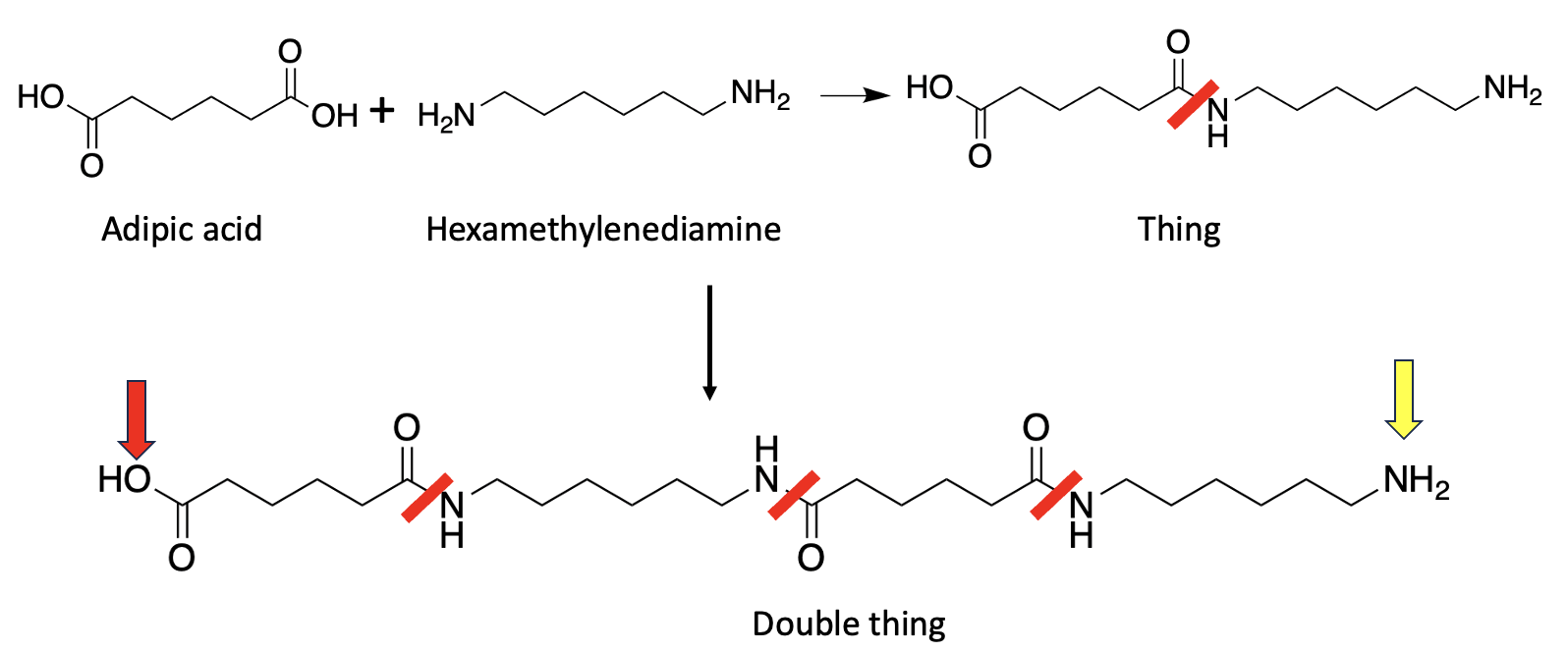
Figure 2. (Top) Adipic acid reacts with hexamethylenediamine to form an amide bond (red line), just like the reaction in Figure 1, but there's a big difference. The reaction product that I've called "Thing" (just to spare you) still contains both an amine and a carboxylic acid, both of which can react with other amines and acids on from molecules. Two molecules of Thing react in the same manner to form "Double thing," which still contains both the acid (red arrow) and the amine (yellow arrow). This same process continues on and on, forming Nylon. Different types of Nylon contain hundreds or even thousands of repeating units.
Polycyanoacrylates (Super Glue)
Nylon is a polymer consisting of alternating units of two different chemicals, but many polymers repeat units of a single chemical, including polystyrene, polyethylene, and polyvinyl chloride (PVC). The polymer of ethyl cyanoacrylate is called poly(ethyl cyanoacrylate) and is better known as Crazy Glue or Super Glue. The polymerization of ethyl cyanoacrylate is shown in Figure 3.
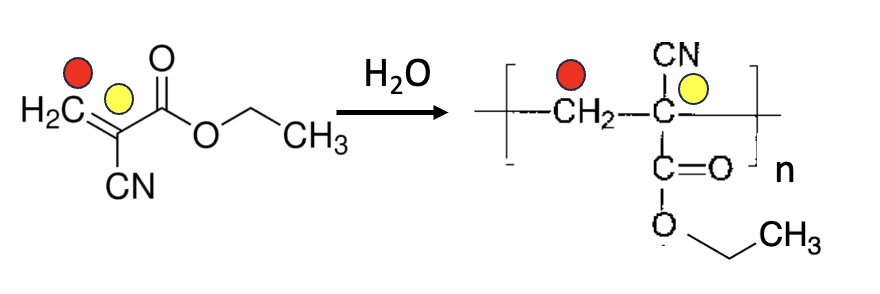
Figure 3. Water promotes the polymerization of ethyl cyanoacrylate (left). (This is why you're supposed to dampen both edges of the pottery, etc., being repaired when you use Crazy Glue.) The red and yellow dots denote the corresponding carbon atoms in the monomer ethyl cyanoacrylate (left) and polymer (right). The letter n describes the number of repeating units in the polymer. This number can be thousands.
Cyanoacrylate polymers are used as "wound glues" (medical adhesives), but they are imperfect for this application:
- Ethyl cyanoacrylate (the monomer) can cause skin irritation and allergic reactions
- Other cyanoacrylate esters are used for wounds (2) because they make the polymer more flexible.
- But all cyanoacrylate esters are cytotoxic and cannot be used internally
- The polymer may be too brittle to hold the wound together
- The polymerization reaction generates heat, which can cause pain, as can the solvent (acetone)
- Removal of the glue is more difficult than for conventional sutures. A solvent (usually acetone) is required to dissolve it.
Organic chemistry to the rescue: Polymers of α-lipoic acid
Polymers of α-lipoic acid have the potential to fulfill the need for versatile and environmentally friendly adhesives, but their performance is plagued by spontaneous depolymerization. We report a family of [stabilized adhesives] that can be tailored for a variety of medical or nonmedical uses...
Messersmith, et. al., Science, Vol 385, Issue 6711, pp. 877-883. 22 Aug 2024 DOI: 10.1126/science.ado6292
But first... Steve steps in with an important public service announcement warning that some pretty hairy chemistry is ahead.

Polymers of alpha-lipoic acid
The Berkely group chose alpha-lipoic acid, a naturally occurring critical biomolecule made by the body, as their substrate for improved wound glue. Lipoic acid polymerizes albeit in a different (and somewhat insane-looking) way (Figure 4).
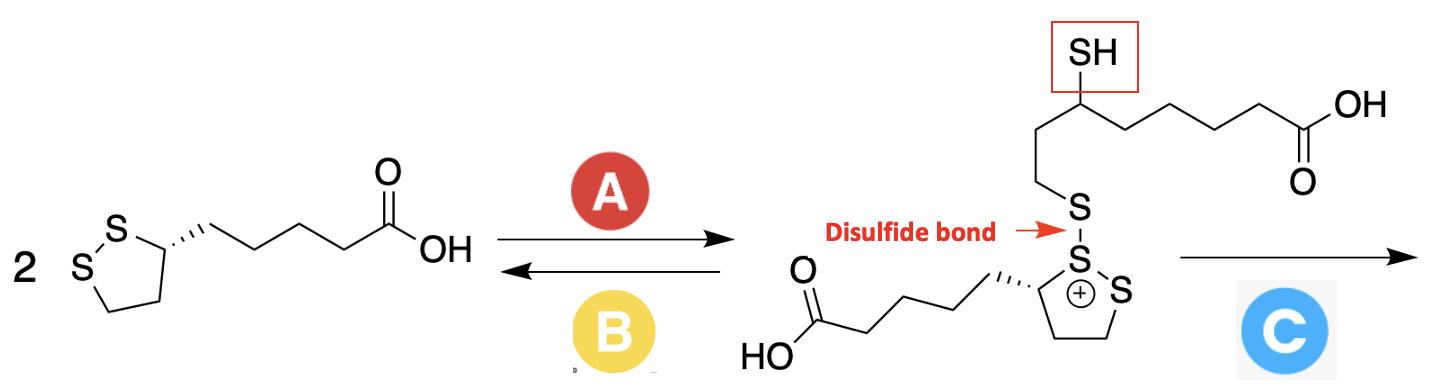
Figure 4. (Left) Two molecules of lipoic acid reacting (Step A) to form a dimer containing a disulfide bond and a free sulfhydryl (SH) group. The sulfhydryl group can then react with a sulfur atom of another lipoic acid, promoting (Step C) the polymerizing. However, steps A and B are reversible, which means that the polymer is inherently unstable, forming and decomposing at the same time.
The group modified the carboxylic acid by reacting it with glycine to form alpha-lipoic acid glycine amide. When this modified compound (4) was added to water, rapid polymerization occurred, but it was different from the polymer from lipoic acid (Figure 5). The authors explain that unlike in Figure 4, this polymer is linked by a thioester bond, which is considerably more stable than a disulfide bond (5). Note that the reaction product has a sulfhydryl (SH) group, which can react to form cross-linked polymers.
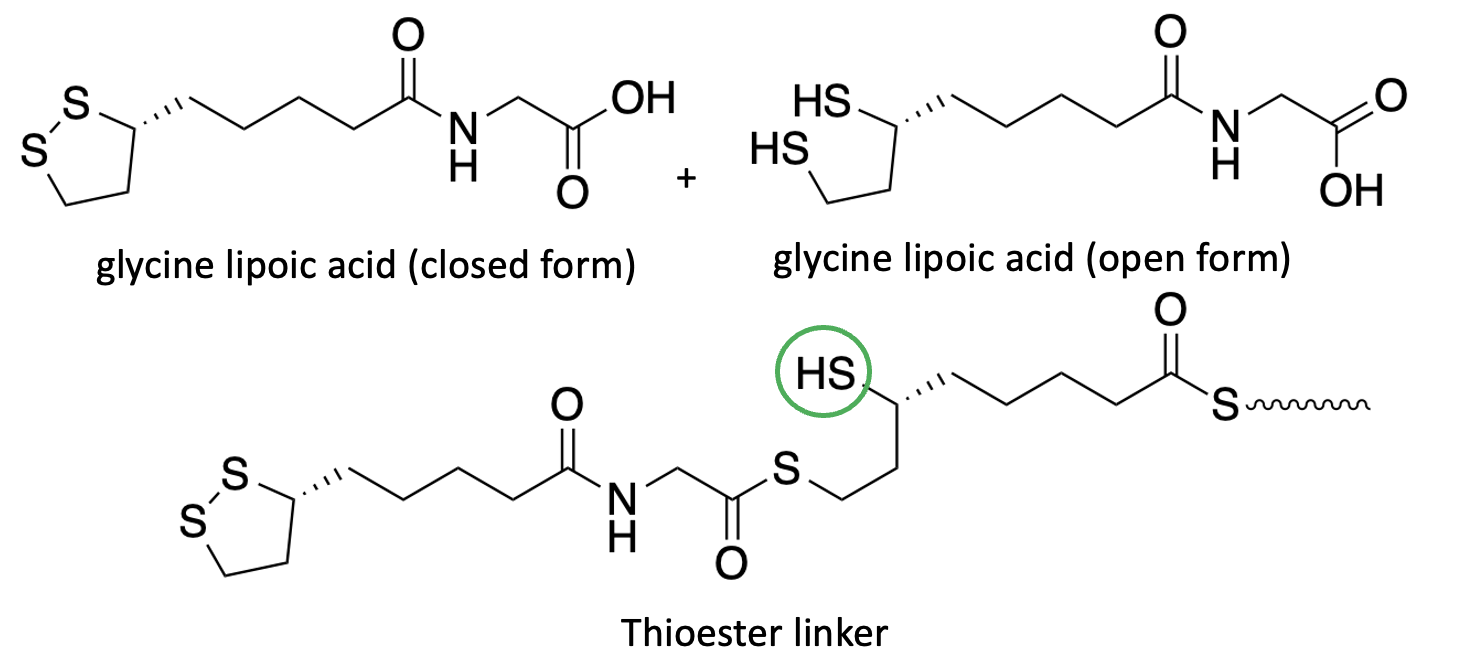
Figure 5. alpha-Lipoic acid glycine amide reacts with another molecule in its open ring to form a thioester, forming the polymer's backbone. Unlike the "natural" polymers in Figure 4, these are not subject to the redox reactions that form and break disulfide bonds and are more stable and suitable for wound closure.
But does it work?
Yes. Figure 6 shows one of the tests that confirms this.
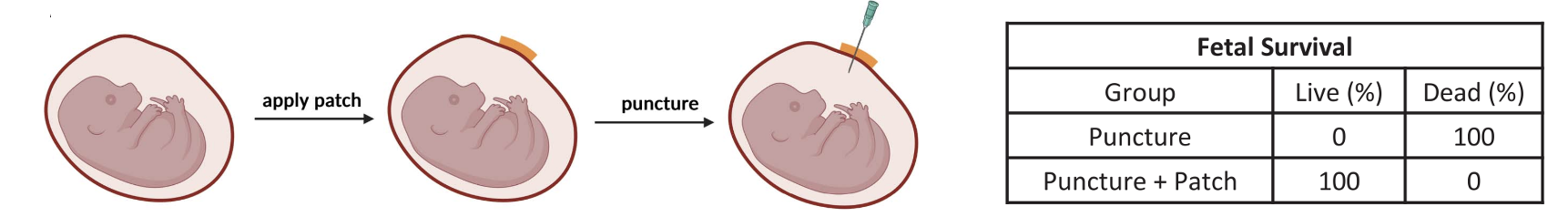
Figure 6. Patches made from the new polymer were applied to mice uteri. After about two weeks, the patches remained intact, and 100% of the fetuses grew normally. The "glue" was well tolerated by the mice. When the gestational sacs were punctured with a needle, the sacs that were pre-sealed with polymer patches lost about 50% less amniotic fluid than unsealed sacs, showing that the patches effectively reduced fluid leakage. In the unsealed group, none of the fetuses survived by day 18. In the pre-sealed group, 100% fetal survival was observed. Credit: Messersmith, et al., Science, 22 Aug 2024, Vol 385, Issue 6711, pp. 877-883. DOI: 10.1126/science.ado6292
Bottom line
This is a quintessential example of how organic chemistry can address and solve a real-life problem. By using a clever strategy to modify a flawed medical adhesive, the Berkely scientists could identify, address, and solve a problem that could lead to a product that could benefit surgical patients.
Pretty cool if you ask me. Steve and Irving may or may not agree.
NOTES:
(1) The polymer I showed was Nylon 6,6, meaning that there are six atoms from the dicarboxylic acid and 6 from the diamine. Choosing different starting materials gives different Nylons with different properties. For example, Nylon 4,6 is made from tetramethylenediamine (4 carbon atoms) and adipic acid (6 carbon atoms) and has a higher melting point and improved thermal stability compared to Nylon 6,6, making it suitable for high-performance applications such as automotive parts and electrical components.
(2) Two other monomers, octyl acrylate, and butyl acrylate, can be polymerized similarly, but the resulting polymers have different physical properties that make them more suitable for medical use.
(3) To those of you with limited organic chemistry under your belts, that five-membered ring containing two sulfur atoms looks bizarre, and its reaction with another one even more so. I'd rather swallow a pound of #6 nails than go into this. You're just gonna have to trust me.
(4) Carboxylic acids and amines themselves do not react to form amides. Instead, a process activates the carboxylic acid, making it susceptible to amide formation. I don't get paid enough to broach this.
(5) Disulfide bonds can easily be broken by endogenous reducing agents, especially glutathione, the most prevalent antioxidant in the body.



