Autumn arrived yesterday, and many of you are probably looking forward to taking a drive to stake in the spectacular tree colors. But, I suspect very few of you are looking forward to a Dreaded Chemistry Lesson From HellTM that will explain the origin of these colors. Sorry, you gotta take the good with the bad.
Of course, neither Steve nor Irving gets to experience fall (or any other season down in hell), but how about if we give them a break? Let's bring the old cranks up for a bit and see if they can help explain why leaves come in such a variety of colors depending on the season. It's all chemistry.

Although Steve (left) and Irving are perpetually miserable individuals even they can have a joyful moment. Both are big fans of The Mamas and the Papas (especially Michelle Phillips). This, plus the advent of autumn, got them, albeit temporarily, into a rare festive mood.
To demonstrate why leaves change color, there's a cute experiment I used to supervise when I was a teaching assistant at UVA while trying to make sure that pre-med students didn't blow themselves up in organic chemistry lab. It’s called thin-layer chromatography (TLC).
Here's how it works.
TLC plates (Figure 1, Left) are typically glass with a silica gel coating, which acts as a medium for the pigments to separate. A tiny amount of a compound or mixture of compounds is applied to the plate about one-quarter of an inch from the bottom. Then the plate is immersed in a solvent (there are several common ones) inside a developing chamber, which can be a covered jar, beaker, etc. As the solvent runs up the plate the compound(s) at the origin move along with it. Different compounds move at different rates, depending on their polarity, and this enables the chemist to identify how many different chemicals are in the mixture and (often) what they are.
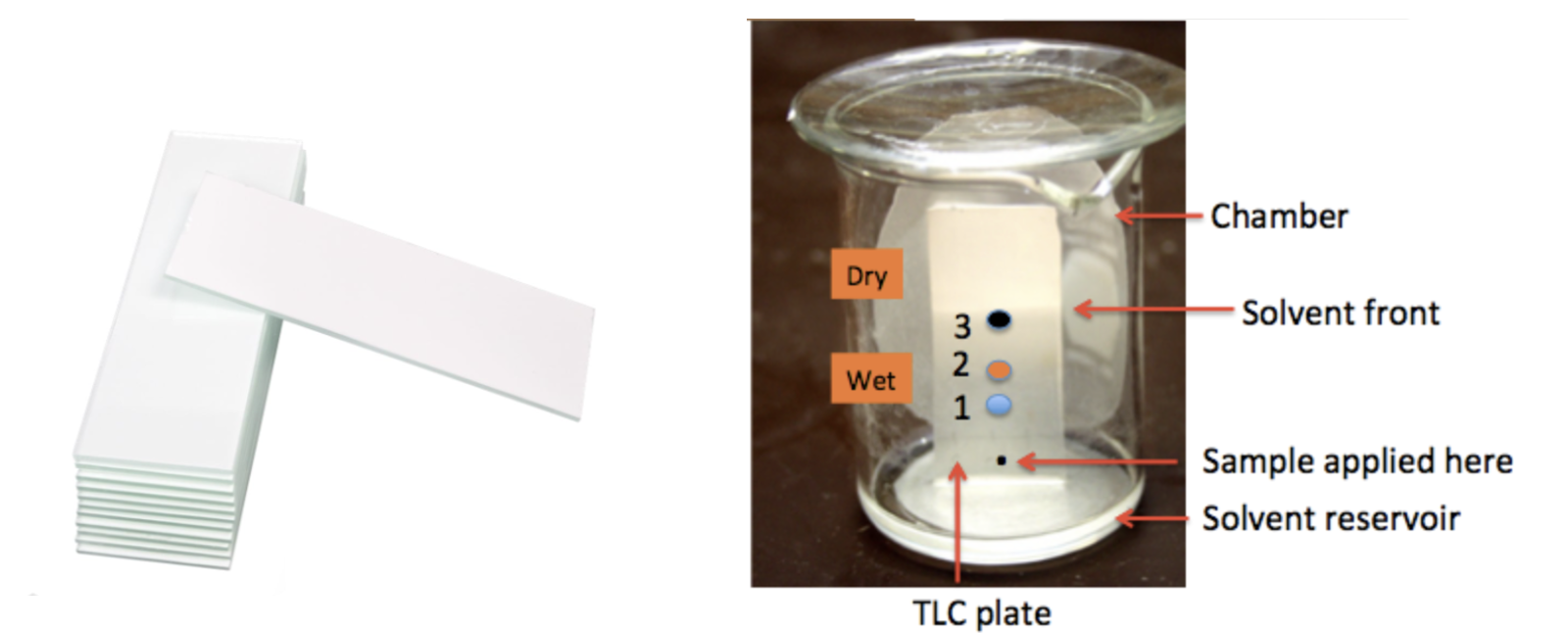
Figure 1. (Left) Silica gel TLC plates (Sigma-Aldrich catalog). (Right) A typical TLC development. The small black dot near the bottom represents the crude mixture to be separated. You can see that upon development that three different compounds are present, blue, orange, and black in decreasing order of polarity. This is a picture, not a real plate. In real life the spots don't usually separate so cleanly, nor are they perfectly round or colored like this.
Using TLC to see leaf colors
Rather than leaves, spinach is used because it contains more pigments. The leaves are ground up with a mortar and pestle in the presence of a solvent and sand; the solvent extracts the leaf pigments. In the example shown in Figure 2, the mixture is applied as a thin band (rather than a spot) to the plate, which is put into the developing chamber. Much as before, the solvent moves up, it carries the pigments with it, separating them into colorful bands, but note the number of different compounds and their colors. This simple experiment shows us the different pigments found in leaves—orange, yellow, and green—the same colors we see as the seasons change.

Figure 2. Thin layer chromatography of spinach extract. The chemical structures are shown to the right of each component.
Polarity, briefly
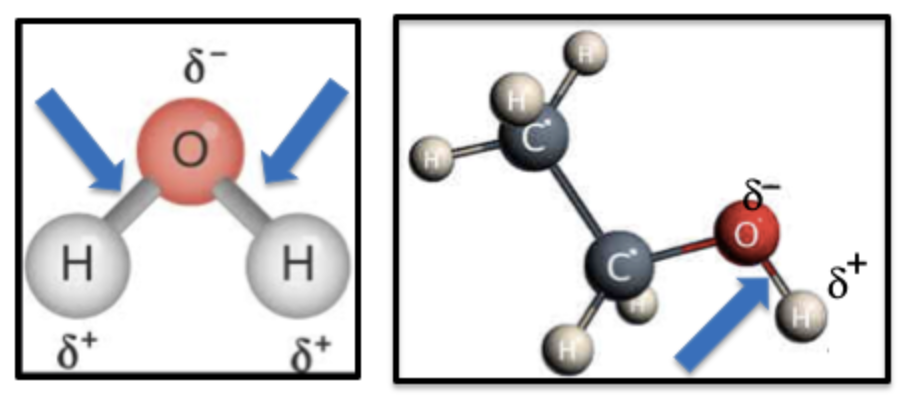
Figure 2. Polarity in chemistry means that a bond has an unequal distribution of the shared electrons between two bonded atoms, causing a small positive and negative charge (think magnets). The bonds (blue arrows) in water (Left) and ethanol (Right) are polar because oxygen atoms have a strong attraction to electrons, pulling them closer (this is because of electronegativity), making one side of the O-H bond slightly positive and the other slightly negative.
By contrast, carbon and hydrogen atoms share electrons equally because they don’t have a strong pull on them. These bonds are non-polar. This is the basis of "like dissolves like" – the reason why water and alcohol mix but water and oil do not. Alternatively, polar substances are attracted to other polar substances; the same holds true for non-polar substances.
Polarity and separation
The position of the pigment bands shows how 'sticky' they are to the plate. The less they stick, the farther they travel. It tells us about the properties of the pigments, specifically, how polar they are.
ß-Carotene is the orange band at the top, just where it should be. The chemical is composed entirely of carbon and hydrogen, just like oil, gasoline, and kerosene, all non-polar, and goes flying up the plate. Atoms like oxygen or nitrogen increase polarity, making the compound stick more strongly to the silica (specifically the oxygens bound to silicon). Chlorophyll (green), which contains both nitrogen and oxygen atoms, is found near the middle of the plate, meaning that it is more polar than ß-carotene and less so than lutein.
Lutein, a yellow compound in the xanthophyll family, contains only two oxygen atoms, but is the most polar. This is due to the presence of two hydroxyl (O-H) groups)). Hydroxyl groups are strongly attracted to other hydroxyl-containing molecules like silica.
Why do leaves change color?
Well, it's "Monday, Monday" (another of Steve and Irving's favorites) night, and this means it's time to watch the Yankees, a different kind of hell, as they limp into the playoffs and get ousted in the first round, perhaps by Houston. Yes, it's time to be tortured once again—probably how you guys feel after reading this.
NOTES:
(1) Synthetic organic chemistry is a technique for converting one chemical compound to another. This is precisely what Walter White did when he converted Sudafed to methamphetamine.
(2) There are many more chemical compounds present in spinach. Some are not colored, so they can't be seen. Some are present in amounts too small to see. And there can be bands within each band that just happened to run up the plate the same distance (co-elution).





