If asked to name a single chemical found in coffee roughly 100% of you would answer 'caffeine.' And you would be correct.
Caffeine is a member of the methylxanthine family of alkaloids. An alkaloid is defined as any naturally occurring, nitrogen-containing chemical, in plants. There are thousands of them out there, and most of them have a "basic nitrogen." Most alkaloids are toxic. This makes sense since the primary purpose of alkaloids is to protect the plant that biosynthesizes them. This explains why you see a lot of vinca planted in areas where deer are present.

Vinca aka periwinkle is not eaten by deer because it contains toxic alkaloids. Photo: Saunders Brothers

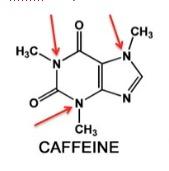
Figure 1. Three common methylxanthines. Photo: Richard J. Miller
Despite being an alkaloid, caffeine isn't especially toxic. It is one member of a class of alkaloids called methylxanthines (Figure 1). One molecule of caffeine (left) contains three methyl groups attached to nitrogen (red arrows). Theophylline, an old asthma drug has two. The green circle shows where the "missing" methyl group is relative to caffeine. Theobromine, a component of chocolate, also has two methyl groups but they are found in different positions compared to those in theophylline. In this case, the methyl group that is found in caffeine is absent (blue circle). Theobromine and theophylline are isomers.
Let's say that for whatever reason, probably to get some sleep, you don't want caffeine in your Joe. Have you ever considered how companies get rid of it (1)? If not, you'll probably be surprised. It's all chemistry, and some of it is purely crazy.

This is not how you get decaf coffee. Photo: XFrogs
Caffeine is not very soluble in water (about 2 grams per 100 mL at room temperature) so if you give your beans a water bath nothing much will happen. On the other hand, caffeine is very soluble (66 grams per 100 mL) in boiling water. Even the nincompoops at EGW will probably know that if you pour boiling water through ground-up coffee beans just about everything that makes coffee taste like coffee will also go along for the ride leaving you with coffee grounds. So, how do you get rid of the caffeine?
To a chemist it's obvious - choose another solvent, one that will dissolve the caffeine and leave the other stuff alone. This is exactly how the most common commercial processes of manufacturing decaf coffee work. This technique is called extraction - the separation of two or more chemicals by making use of the difference in their solubility in various liquids. We chemists use it almost every day. And so do most coffee manufacturers.
Two solvents that are typically used to extract caffeine from coffee are ethyl acetate and methylene chloride, aka dichloromethane. If there is a better demonstration of the silliness of the artificial divide between 'organic' and 'not organic' I've yet to see it.
Let's go to the Organic Facts website and see what they have to say.
[The process [used to make decaf coffee] is extensively used in the coffee industry and can be both organic and inorganic. In this process, the coffee beans are first treated with hot water and then with Methylene Chloride or Ethyl Acetate.
OK, so far so good.
Thereafter, the Methylene Chloride or Ethyl Acetate is dried and the coffee beans are again subjected to hot water. If this process involves Methylene Chloride, then the product turned out is invariably inorganic, since Methylene Chloride can only be prepared artificially.
Now, this is beginning to sound disturbing. But then...
However, if the process involves Ethyl Acetate, then it gives Inorganic Decaf Coffee if the Ethyl Acetate used in it was synthetic (synthesized artificially) and Organic Decaf Coffee if that Ethyl Acetate was obtained from natural sources (which it can be).
NO!!! This is just plain nuts. A chemical is a chemical, no matter where it comes from. There is no difference. Except for (maybe) price. You might as well just slap an organic label on the coffee bag depending on whether it's packaged on an even or odd numbered date.

So, if you're buying organic decaf coffee that is prepared by extraction (2), know the following:
1. Synthetic ethyl acetate is identical in every way to "natural" ethyl acetate.
2. Ethyl acetate, regardless of its source, boils at 77° F. The temperature at which coffee is roasted is about 450° F. Virtually all of the ethyl acetate, which won't harm you anyhow, will be long gone.
Either way, don't let it keep you up at night.
NOTES:
(1) Standard methods remove about 95% of the caffeine, not all of it.
(2) There are other methods of removing caffeine from coffee, including water/filtration, supercritical carbon dioxide (expensive), and coffee oil. In the US, the water method is gaining steam. Sorry about that. Most decaf coffee is still made using the extraction method.