
The monster explosion that destroyed a good bit of Beirut was caused by the detonation of more than 2,700 tons of the fertilizer ammonium nitrate – a ghastly amount of one seriously scary chemical. If the name sounds familiar it's because the chemical is notorious. It also destroyed Texas City in 1947 and the Alfred P. Murrah Federal Building in Oklahoma City in 1995.
It's no accident that ammonium nitrate is an explosive; it has much in common with other well-known explosive chemicals:
- A large number of nitrogen and oxygen atoms and a small number of carbon atoms.
- Functional groups containing atoms that can form stable gases, for example, nitrogen, oxygen, water, ammonia, often violently, when the explosive molecule decomposes. You can think of these gases as being "trapped" within the molecule.
Those trapped gases are none too happy about being crammed together in a little molecule. No sir, they want their freedom and it can take a very small nudge, physical or chemical, to grant them their wish. Let's take a look at some explosive chemicals. You should see a pattern.
1. Triaminotoluene (TAT) vs. trinitrotoluene (TNT)

TAT contains 7 carbon atoms (C) and 3 nitrogen (N) + oxygen (O) atoms. It is not explosive.
TNT also contains 7 C but 9 (N + O) atoms. It also has three nitro groups, which are itching to escape as carbon monoxide, nitrogen, and water. And you get... quite a bang for your buck. Two molecules of TNT decompose to give 15 molecules of gas – three of nitrogen, five of water, and seven of carbon monoxide. That's an awful lot of gas trapped in one molecule, which is why it goes boom when enough energy (heat, shock, or electricity) is supplied to get it started. Here is the equation.

(s) means solid (g) means gas
Source: Bristol University
2. Pentaerythritol vs. Pentaerythritol Tetranitrate (PETN)
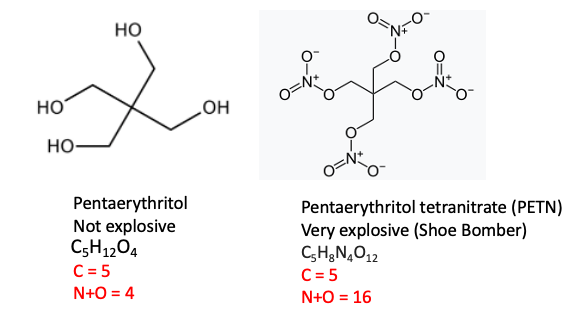
Pentaerythritol, a common chemical intermediate for the production of plastics, paints, and cosmetics. You could fire it out of a bazooka and it won't do anything. But when the hydroxyl groups are converted to nitrate esters you have a completely different beast. Pentaerythritol tetranitrate contains 29 atoms; 16 of them are nitrogen or oxygen. This makes it a scary explosive. It's the chemical that Richard Reid ("The Shoe Bomber") had in his shoe in an American Airlines flight to Miami in 2001. Fortunately, he was too incompetent to ignite it.
3. Triacetone triperoxide
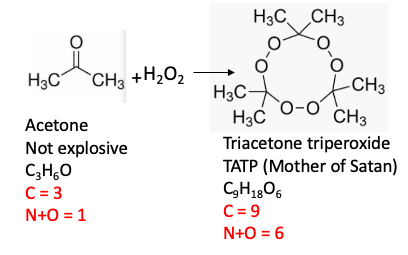
Triacetone triperoxide belongs to a class of compounds called organic peroxides. Smaller (low molecular weight) organic peroxides contain a high proportion of oxygen to carbon, which makes them very dangerous. This one is no exception; its nickname, Mother of Satan," is well-deserved. Ether (diethyl ether) is one of the most common organic solvents used in labs. It comes in a metal can with a label on which you need to write the date the bottle was first opened. Once the bottle is opened the ether is exposed to oxygen and it slowly forms explosive hydroperoxides. Sometimes just opening the bottle can set off an explosion This is why bottles of ether older than 6 months must be discarded.
4. Ammonium nitrate
NH4NO3
C = O (N+O) = 5
In some ways, ammonium nitrate is the perfect explosive. It contains no carbon - only nitrogen, hydrogen, and oxygen. It's a white solid made up entirely of gases. When heated it is only too happy to give them up.
NH4NO3 (s) -------> N2 (g) + 2H2O (g) + 1/2 O2 (g)
When a single molecule of ammonium nitrate decomposes it gives up 3.5 molecules of gas (1).
So, without going into thermodynamics (neither of us wants this) the take-home message here is that when a lot of gas is released quickly from a molecule packed with it bad things happen.
But not always. The self-inflating life vests in airplanes work in a similar manner. Compressed carbon dioxide is stored in canisters in the vest. When they are opened (either manually or automatically by exposure to water the vests inflate rapidly.
Just don't pull the cord in the plane.
NOTE:
(1) The reaction isn't this simple. Other gases, notably nitrogen dioxide, are also formed. This is the reason for the orange cloud.



