Ask anyone, whether it's a librarian, vegetarian, Hungarian, or a dromedarian (yes, I made that up), or anyone else, where the first successful method of preventing norovirus (aka "stomach flu") infections might come from, and exactly none of them will answer: Llamas. (The few that guessed this were immediately locked up.)
But there is an unusual immune component found in llama blood, a mini-antibody called a nanobody, that seems to be effective in neutralizing GII.4 HuNoV, the norovirus strain that is responsible for most outbreaks of the infection. Although there is plenty of vaccine research encompassing multiple strategies, there are no norovirus vaccines (or anything close to one, in my opinion) or antiviral drugs to prevent or treat the infection.
In short, you're doomed; catching the bug doesn't prevent you from getting it again, and norovirus is insanely infectious, requiring only 10-100 viral particles – a minuscule exposure – to keep you at an intimate distance from a toilet for a day or two. Perhaps worse is that the little bastards are hard to kill (2), scoffing at alcohol sanitizer – something I wrote about recently.
A little English, anyone? What does GII.4 HuNoV mean?
- "G" refers to Genogroup – a family of viruses consisting of multiple strains that may or may not be genetically similar. There are 10 known norovirus genogroups.
- Roman number "II" is one of the ten (the second, duh) genogroups designated GI through GX.
- A genotype is a subset of a genogroup where there is more genetic similarity of the viruses within the classification. There are 49 known genotypes among the 10 genogroups.
- HuNoV means human norovirus.
- "4" refers to one particular strain, which is a major pain in the ass. It is responsible for most outbreaks. It readily undergoes genetic changes, creating new variants. The variants of GII.4 have different viral capsids (each presenting different antigens), which is how the virus evades the immune response. This is one reason why a vaccine approach has failed so far. We don't like GII.4 one bit.
Ready for some llama drama, mamma?
Feel free to read "A single nanobody neutralizes multiple epochally evolving human noroviruses by modulating capsid plasticity," which was recently published in Nature Communications if you wish. Don't wish. You'll off yourself before page 2. Hopefully, you won't react similarly to my Cliffs Notes version.
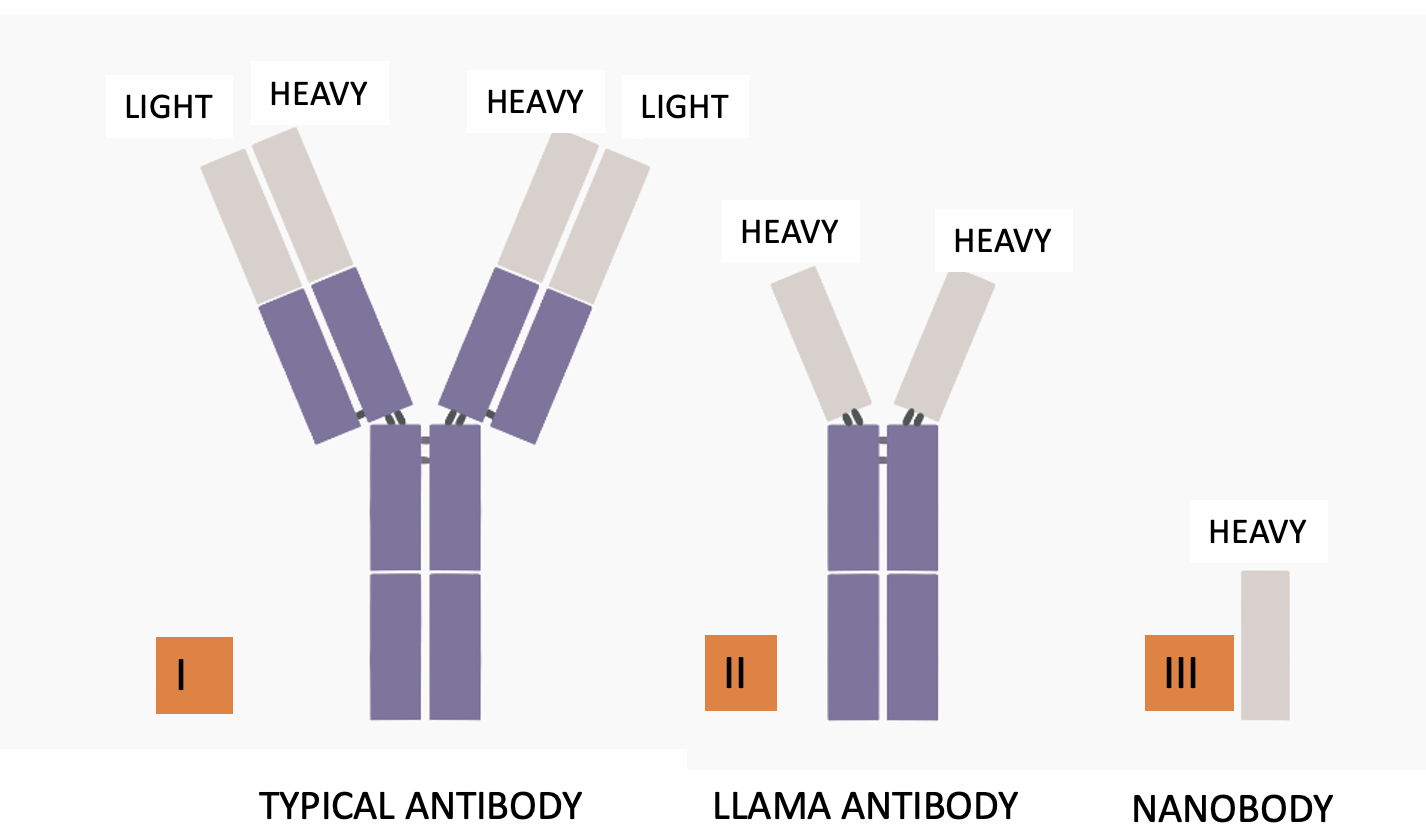
Figure 1. (Left) A typical antibody (I) has a Y shape. It is made up of two heavy chains and two light chains. Heavy chains determine the function of the particular antibody. Light chains function in antigen recognition, but they mutate like madmen. Mutation of the antibodies formed in response to norovirus infection is why there is little protection against a subsequent infection. (Center) Camelid (llamas, camels, and other vile beasts) antibodies contain only the heavy chains (II), making them more stable (3). (Right) Nanobodies can be formed from camelid antibodies (III). You don't want to know the details. They are small, stable, and have a high affinity for viral antigens. Original Source: Science Focus (The Hong Kong University of Science and Technology)
Advantages of nanobodies in neutralizing norovirus
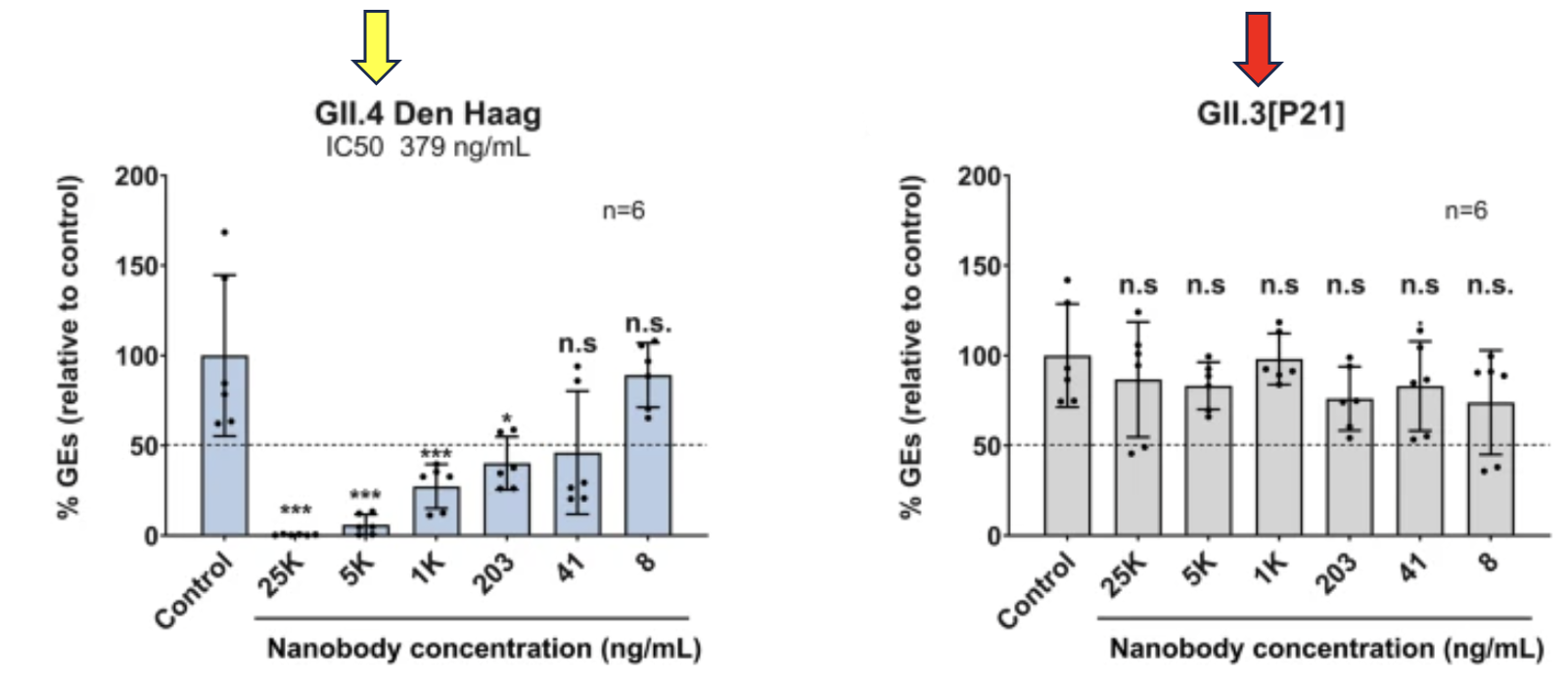
Figure 2. On the graph to the left - GII.4, the norovirus strain that causes the most infections, in cultured cells with different concentrations of the nanobody (named M4). The Y-axis measures %GEs (viral genome equivalents) – a measure of the decrease in viral replication. The X-axis shows the concentration of M4 added to the cell culture. The graph shows a possible (4) dose dependence, e.g., the higher the concentration of M4 the greater the reduction of GE. On the graph to the right - There is no apparent inhibition of genotype GII.3 regardless of the concentration of M4, so M4 would not be expected to generate a protective immune response to norovirus.
Why nanobodies?
Let's let the authors answer this.
Nanobodies have several advantages over traditional antibodies for their development as immunotherapeutic agents. They are smaller in size (~15 kDa), exhibit higher stability over a wide range of temperatures, and are resistant to protease cleavage.
Prasad, et. al., Nature Communications DOI: 10.1038/s41467-023-42146-0
Bottom line
It would seem that when it comes to generating an immune response to HuNoV, smaller is better. And an added advantage is the absence of the light chains that cause rapid mutation, one of the problems that has plagued vaccine development. I have only scratched the surface of this study. The virology is accompanied by (among other things) a discussion of the mechanism of the binding of M4 to HuNoV, even down to the atomic level as determined by X-ray crystallography. Like this:
Figure 3. Molecular interactions involved in the binding of GII.4 to M4. Don't try to understand this. Just enjoy the pretty colors. (All kidding aside, the science here is kind of amazing.)
There are or have been 48 clinical trials in an effort to discover an effective immunological agent to prevent or modify norovirus infection, including five vaccines in various stages of development. None of them looks especially exciting. Perhaps this group has come up with a different kind of beast.
NOTES:
(1) No - llamas don't have humps but I couldn't resist making a stupid joke.
(2) You can't really kill viruses because they are not alive. The term is commonly used anyhow.
(3) The use of animal immunity to treat human disease is not new. There's a history of animal immune sera being administered to humans -- e.g., equine immune serum against diphtheria.
(4) Some of the data lack statistical significance, so while the graph looks like the nanobody is inhibiting the virus in a dose-dependent manner, this cannot be concluded.




