A new press release from MindMed, a biopharmaceutical company geared toward developing drugs to treat brain health disorders, is intriguing. The company has been running clinical trials on LSD as a potential therapy for generalized anxiety disorder (GAD). It seems to work:
“I’ve conducted clinical research studies in psychiatry for over two decades and have seen studies of many drugs under development for the treatment of anxiety. That MM120 [LSD 100 micrograms] exhibited rapid and robust efficacy, solidly sustained for 12 weeks after a single dose, is truly remarkable...These results suggest the potential MM120 has in the treatment of anxiety, and those of us who struggle every day to alleviate anxiety in our patients look forward to seeing results from future Phase 3 trials.”
David Feifel, MD, PhD, an investigator in the MM120 study.
The FDA agrees. Based on Phase IIb results the agency designated MM120 (a 100 microgram dose of LSD tartrate) as a breakthrough therapy for GAD.
Rather than rehash the limited clinical results I thought it might interesting to examine why a single dose of LSD can provide such a long-lasting effect. LSD stays in the brain long after it has disappeared from the blood – an unusual property for drugs. How is this possible? At least part (if not most) of this effect can be explained by some rather simple organic chemistry. LSD "sticks" in the brain for a very specific reason. It's just like oil and water.
A group from the Department of Pharmacology at the University of North Carolina may have found out why - an unusual mode of binding to a specific receptor in the brain in which the drug traps itself. And, it does this because of some rather fundamental chemistry.
In a 2017 paper published in the Journal Cell, Bryan Roth, M.D, Ph. D., and colleagues provided details of how LSD binds to specific serotonin receptors (1). The details of this binding can be visualized at the atomic level by using a technique called X-ray crystallography, a powerful tool for examining the chemical structures of molecules and how they interact with targets. More on this later.
But first, let's clean up the name because, although LSD is called "acid," it really shouldn't be, at least according to chemists. In fact, not only is there nothing acidic about LSD but it's actually basic. (Anyone know why?) But its precursor, lysergic acid, could rightfully be called "acid" even though it is mostly pharmacologically inert.
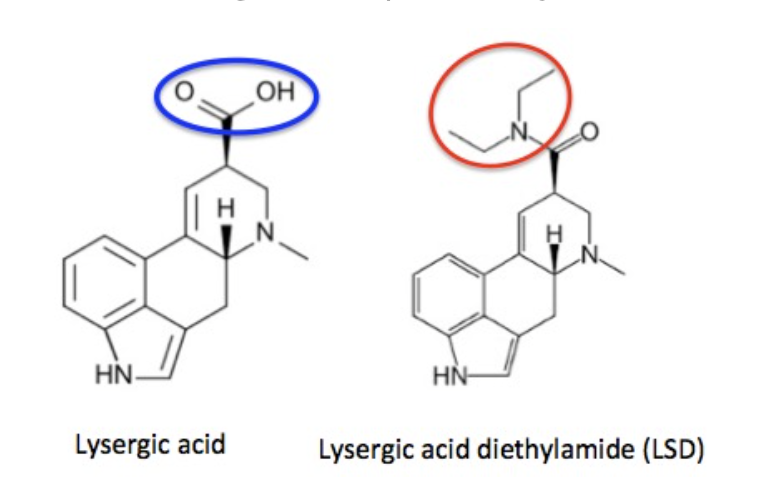
Figure 1. The structures and names of lysergic acid and LSD. The two molecules are identical except for the atoms in the blue and red circles.
Lysergic acid, a natural product which is produced by ergot fungus, has, as the name implies, a functional group called a carboxylic acid (blue circle), hence the name. But lysergic acid is not LSD. LSD is not a natural chemical (3); instead is synthesized in a lab from lysergic acid. The correct name for LSD is LySergic acid Diethylamide. But the term "dropping amide" just doesn't sound right, so users took some psychedelic license.
HOW TO VISUALIZE MOLECULES AND RECEPTORS - X-RAY CRYSTALLOGRAPHY
X-ray crystallography (4) is a science unto itself. It is highly specialized and technical. An organic chemist may be able to understand and use the images that are generated by powerful computers that assemble and process huge amounts of data. But we never really understand the guts of the process, let alone have the knowledge to perform it. This also makes it rather difficult to write about. Here goes nothing.
Roth's group enabled us to see exactly why LSD behaves as it does and it is very cool. The expression "keep a lid on it" is certainly germane here. The image below (Figure 2) shows a simulation of how a very small, but also very critical part of the 5-HT2A serotonin receptor - the target of LSD works (5). The light blue represents a portion of the receptor - the binding site. The dark blue area is an unusual structure called a lid. As the name implies, the lid can either let something out or keep it in. In the closed form (left) nothing is getting in or out of there. In the open form (right) you can see a molecule (red) that is looking rather eager to escape. LSD keeps the lid closed, which is why it stays in your brain longer than you might expect.]
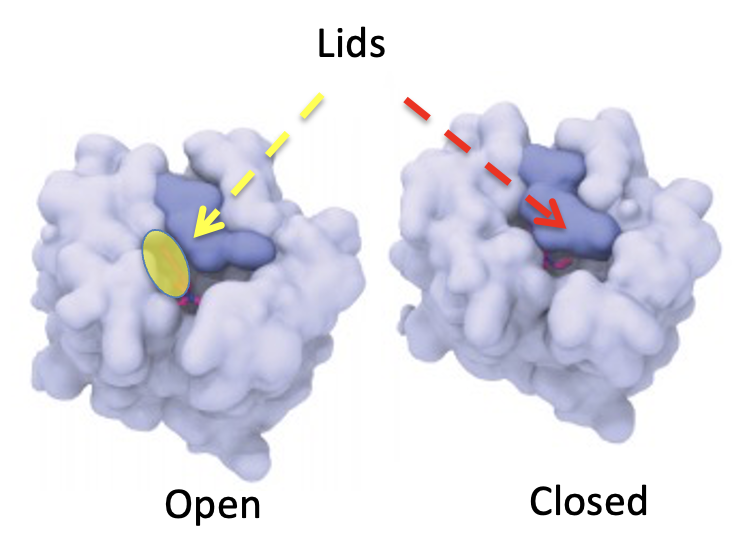
Figure 2. The serotonin receptor that binds LSD. (Left) Open form. The yellow oval represents the space in the open form that serotonin agonists or blockers can enter and exit. (Right) Closed form. The same receptor with the lid being held closed by LSD, trapping the drug inside the receptor. Source: Cell
If this isn't cool enough, let's take a closer look, all the way down to the atomic level. Figure 3 shows the precise interaction between the diethylamide portion of LSD and a single amino acid part of the receptor, the amino acid leucine (2). LSD "finds" its serotonin receptor and attaches to its binding site in such a way that the diethylamide from LSD and leucine from the protein are in close proximity. The interplay between the diethylamide part of LSD (pink) and the leucine fragment of the lid protein (red) is shown by the green arrow. They attract each other.
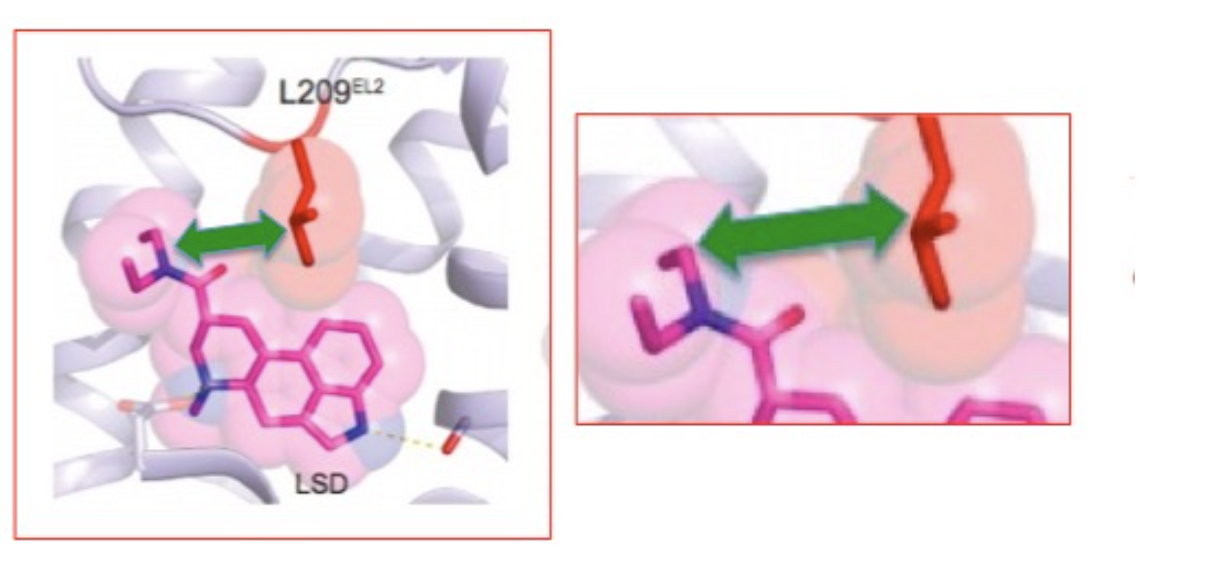
Figure 3. (Left) The interaction (green block arrow) between the diethylamide part of LSD (pink) and a leucine component of the receptor (red). (Right) A closeup of the same interaction. L209 refers to the 209th amino acid in the protein chain, which consists of amino acids chemically bound to each other. "L" is shorthand for leucine. Source: Cell
Why should the diethylamide and leucine attract each other? It's Chemistry 101 – like attracts like. Figure 4 shows the chemical structure of both fragments and why they "like" each other. Amino acids can be broadly categorized as "hydrophobic" aka lipophilic – hates water but likes oil and "hydrophilic" (likes water but doesn't like oil). "When the amino acid side chain contains only carbon atoms in its group, it is considered hydrophobic. Leucine (blue circle) is one of these. The four carbon atoms in the diethylamide part of LSD are also hydrophobic. Like attracts like, so these two groups attract each other. It is this attraction that holds the lid closed. Conceptually this is no different than gasoline or oil and water. This is also the exact same reason why red blood cells become distorted and function poorly in people with Sickle Cell Anemia, something I recently wrote about.
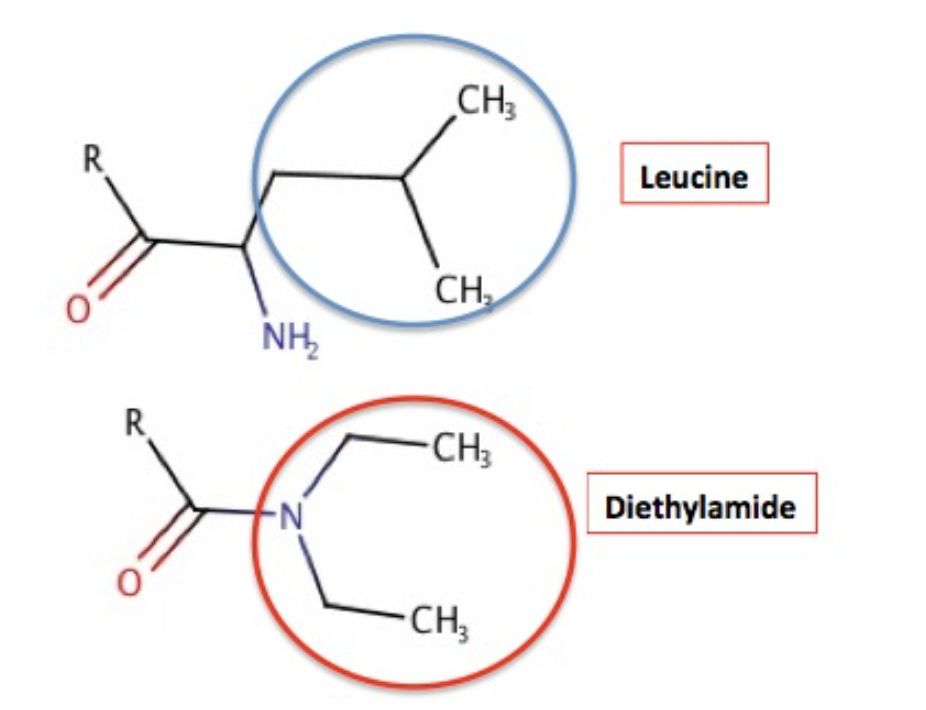
Figure 4. (Top) Leucine contains four hydrophobic carbon atoms - they prefer oil to water. (Bottom) The diethylamide fragment of LSD also contains four hydrophobic carbon atoms. This is why they "stick" together.
It is well-known by chemists that the properties of a drug can vary greatly depending on only a handful of atoms. It would be difficult to come up with a better example.
NOTES:
(1) There are 14 subtypes of serotonin receptors, found in different locations throughout the body, not just the brain, and they produce a wide array of responses. For example, Zofran, a drug invented to control chemotherapy-induced nausea and vomiting acts by blocking (an antagonist) the type 3 receptor (5-HT3) while Prozac acts as an agonist for the 5HT2C receptor.
(2) Proteins are long chains of amino acids chemically bonded together. The properties of the protein depend greatly upon the characteristics of the amino acids. There are 20 different amino acids that make up proteins, and they have very different properties. These different properties are responsible for the enormous number and variety of proteins.
(3) The classification of chemicals as "natural" vs. "synthetic" is a distinction without a difference. As we at ACSH have maintained forever, it makes no difference where a chemical comes from. The structure of the chemical and its properties determine its pharmacological profile, not its origin.
(4) ChatGPT: "X-ray crystallography is a technique used to determine the atomic and molecular structure of crystalline materials. It involves exposing a crystal to a beam of X-rays, which are scattered by the electrons in the crystal lattice. By analyzing the pattern of scattered X-rays, scientists can determine the arrangement of atoms in the crystal. This information is crucial for understanding the properties and behavior of materials at the atomic level. X-ray crystallography has wide-ranging applications in chemistry, biology, physics, and materials science, and has played a key role in advancing our understanding of the natural world."
(5) 5-HT is short for 5-hydroxytryptamine - another name for serotonin.
Original Paper: Cell, Volume 168, Issue 3, p377–389.e12, 26 January 2017; https://doi.org/10.1016/j.cell.2016.12.033



