
For many years, norovirus vaccine (NoV) research has been going gangbusters but with nothing to show. NoV is one of the primary culprits behind acute gastroenteritis worldwide. This miserable, super contagious bug causes severe diarrhea, and vomiting, which can lead to dehydration; this can be particularly dangerous for young kids, the elderly, and those with weakened immune systems. Although merely hideous for most of us, NoV causes about 200,000 deaths globally per year, about one-quarter of those being children under the age of 5.
To make matters worse, it spreads like wildfire in places where people are close together, like schools, nursing homes, and cruise ships. An effective vaccine would be a huge advance in public health.
But this is easier said than done. The ClinicalTrials.gov site lists 49 clinical trials, approximately zero of which have been successful. That number remains at zero following yet another failed trial.
If you had hopes of avoiding another norovirus season, HilleVax, a biotech focused on discovering and developing novel vaccines, just dashed those hopes. The Boston-based biotech just announced the results of clinical trials of its experimental norovirus (aka the "stomach flu") vaccine, HIL-214 (licensed from Takeda), in infants. Aside from unexpected adverse reactions (there were none), the news couldn't be worse. The vaccine provided no protection from norovirus infection. Zilch. Speaking of upset stomachs...
The 5-day chart of the stock price is perhaps just as stomach-churning for those who own it:
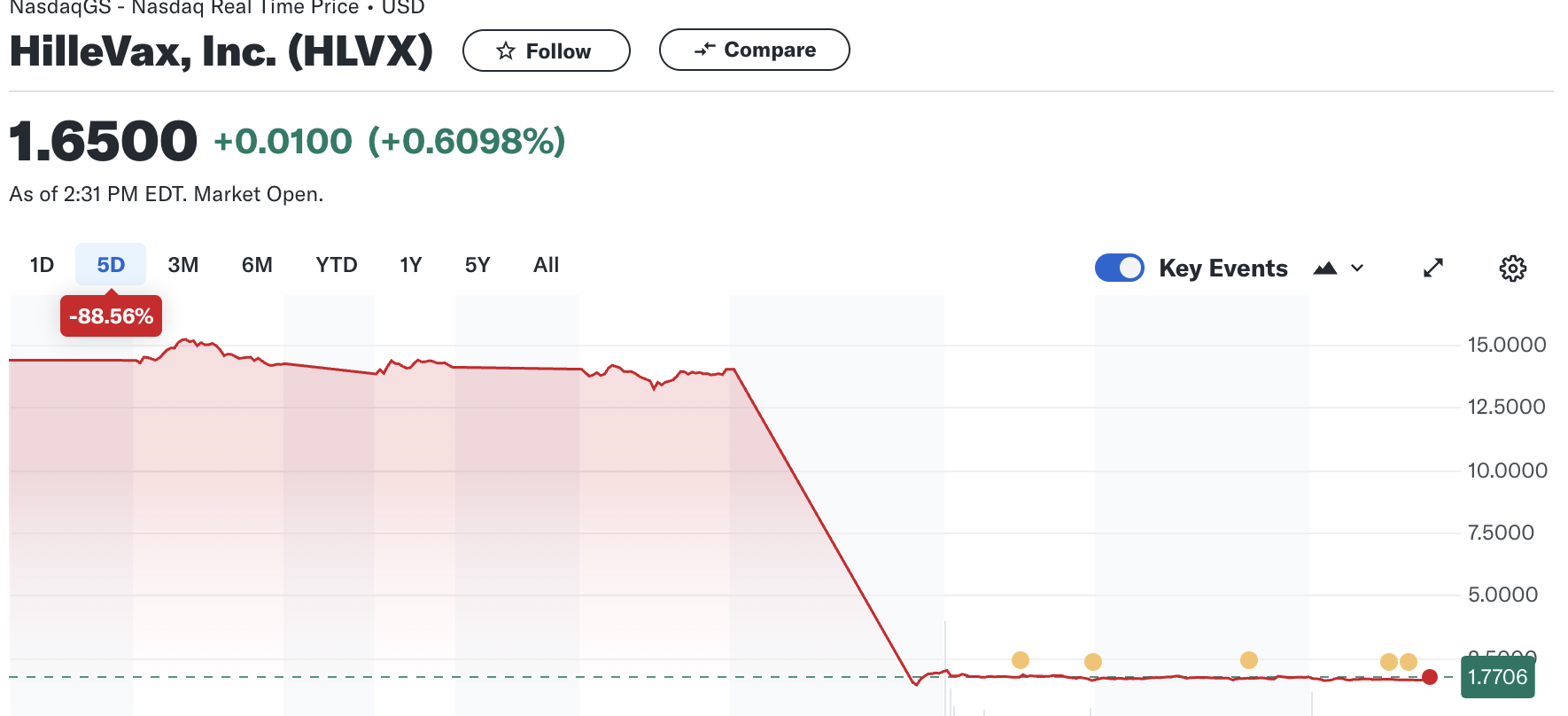
Its 52-week high was $20.22. For those of you who are not well-versed in the stock market, $20.22 is much more than $1.65, something this guy found out.

In retrospect, these results are disappointing but not surprising. The phase 2B challenge study (1), which was called NEST-IN1 (Norovirus Efficacy and Safety Trial for INfants), tested the ability of the company's HIL-214 vaccine to prevent or lessen the effect of NoV symptoms.
Norovirus is a tough nut to crack
The primary reasons that HIL-214 didn't live up to expectations are:
- Although the development of a NoV vaccine is receiving plenty of attention worldwide, there is no precedent for its success or obvious path forward. Other clinical candidates, for example, from Vaxart (2) and the Institut Pasteur of Shanghai (3), have both run into trouble.
- Earlier trials on HIL-214 weren't designed to show much. These studies demonstrated only that HIL-214 was safe and could create an immune response. This is a long way from showing efficacy in a challenge trial let alone an approved drug.
What did NEST-IN1 show?
The NEST-IN1 study included a total of more than 1,425 participants and examined a total of 51 (!) endpoint events. The overall efficacy was only 5% (ugh) (95% CI: -64%, 45%). Nor did the study meet its primary efficacy endpoint of moderate or severe acute gastroenteritis due to GI.1 or GII.4 norovirus genotypes – the two strains responsible for most infections. Nor was there any clinical benefit observed across the secondary endpoints. The good news, such as it is, is that the vaccine was safe, and markers of immunogenicity were present.
Bottom line
Another bust. HilleVax is "exploring the potential for continued development of HIL-214 and HIL-216, HilleVax’s Phase 1 ready vaccine candidate, in adults."
Translation: No way.
Any hope on the horizon? This is hard to say but Moderna, which developed one of the messenger RNA COVID vaccines is setting up a Phase 2 trial of a mRNA-based NoV vaccine. Don't count them out.
NOTES:
(1) A challenge study is exactly what it sounds like. Some of the participants are intentionally infected with the virus. My feelings? #### that ####.
(2) Conflict of interest statement: Remarkable, I do not own this piece of s### ---> HilleVax. However, I do own another piece of s### ---> Vaxart. Brown is brown.
(3) The Institut Pasteur decided in December 2022 to suspend its partnership agreement with the Institut Pasteur of Shanghai. Here's the BS corporate-speak given by management: "This choice was made in order to begin a new cycle of conversations for improving the relationship between the two organizations and finding a more productive way to work together." Oh, my sides!



