In 2024 alone, there have been at least dozens – probably hundreds – of news reports on fluoridation, most of which focused on the potential harm of fluoride to developing babies and a possible link to cognitive and behavioral disorders.
Several cities, counties, and states have voted or plan to vote to ban drinking water fluoridation (not fluorination). Often left unstated is the fact that fluoride occurs naturally in groundwater worldwide. A 2017 report by the World Health Organization estimates that 200 million people worldwide are exposed to naturally occurring fluoride levels (from minerals, more on this below) that exceed 1.5 mg/liter, which is considered to be the maximum safe dose, although this number can vary depending on different standards in different countries.
Add fluorinated "forever chemicals," which are a very hot topic—something I wrote about recently (See Why 'Forever Chemicals' are Forever)—and it is not at all surprising that there is massive confusion about the names of these chemicals and their meaning. Hopefully, this article will clear up some of this confusion.
Speaking of which, here's a ghastly example of confusion that was published on the Phoenix News12 site.
It starts out OK:
Though adding fluoride to drinking water is recommended by nearly all public health, medical, and dental organizations — it still doesn't sit well with some people.
But then, this...
Studies have shown that fluorinated water can reduce cavities in children and adults by 25%. Today, about 75% of Americans are drinking fluorinated water, according to the CDC.
News12.com
Hoo boy. This one's a doozie. The correct number is actually zero percent. Drinking water (or any other kind) is never fluorinated – a seriously bad idea by any measure. Fluorine reacts violently with water to form oxygen and hydrofluoric acid, which will eat your face.
The quote above should have used "fluoridated," not fluorinated. Let's see if we can make some sense of these terms.
Fluorine
As I wrote in 2016 (See Fluorine: The Element From Hell), (1) fluorine (F2) – a pale yellow gas – is the most reactive of all elements. It will react, usually violently, with almost every substance on Earth, including a raw chicken, a brick, and charcoal. It will also light a cigarette. (These are rather funny YouTube videos. I urge you to check them out.)
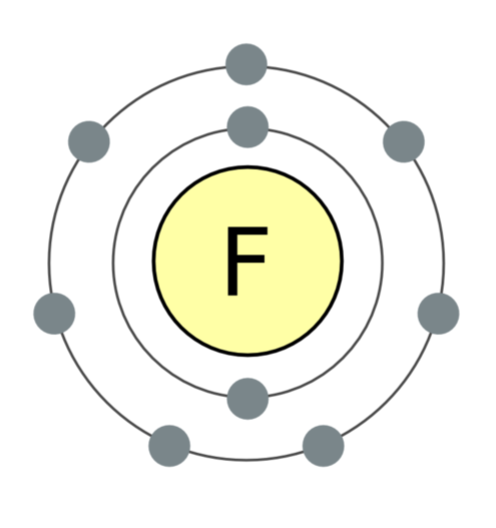
A fluorine atom contains 7 electrons in its outer shell; it desperately wants the 8th, which converts it into fluoride. This is why the element is so reactive. Image: Wikimedia Commons.
Fluorine (the element) exists nowhere on Earth. It is far too reactive. It is not a public health threat since we are never exposed to it. That's good because it will also eat your face.
Fluoride has two meanings
Fluoride the chemical:
Although commonly used, no chemical can correctly be called "fluoride"; it is only half of a name – an abbreviation for a compound that contains a fluoride ion. Unlike fluorine, fluoride (F-) is an anion that must be paired with a counterion (in this case a cation), such as sodium or potassium, to make a "real" chemical.
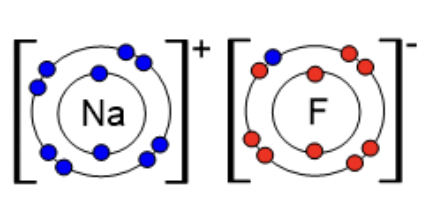
Sodium fluoride is an ionic salt. Fluorine "steals" one electron from the outer shell of sodium, which is only too happy to give it up. Then fluorine becomes negative (F-) while the sodium becomes positive (Na+). When fluoride salts are added to water this process is called fluoridation.
Fluoride the mineral:
There are dozens of fluoride minerals found in the Earth. Fluorite (aka fluorspar) (CaF2) is the most common. It is one of the most color-diverse minerals, the colors coming from impurities (iron, manganese, and yttrium). (Figure 1) These minerals are the source of fluoride in groundwater. And if you ever need some yttrium in your life – and who amongst us doesn't? – now you know what to do.

Figure 1. Fluorite, the primary fluoride mineral, can be many different colors, all beautiful. The top middle image is the mineral under UV light. Images: Flickr, Martin Heigan/Flickr, Wikimedia Commons, Wikimedia Commons.
Bottom line
The terms "fluorine" and fluoride are inherently confusing (as would be expected) to non-scientists. Throw in the fluorinated "forever chemicals," and it's easy to understand the reasons behind this confusion. Whether this article helps with the confusion or adds to it remains to be seen.
NOTE:
(1) This article was technically the first in the Dreaded Chemistry Lesson From Hell® series, although that concept did not yet exist.




