Let's give the DEA and FDA a (Bronx) cheer (1). They continue to amaze. What they may lack in common sense they make up through diversity. As in "They can sure screw up a diverse set of problems."
The DEA keeps bungling. And water is wet
The DEA has shown that it can botch the regulation of an assortment of drug classes. No sir, our mysterious friends cannot be typecast! The sheer variety of the agency's blunders should be admired, if only for completeness and the number and variety of screwups. Here are a few.
- Over-restriction of prescription opioids
- Persecution of (the few remaining) pain management physicians for doing their jobs
- Keeping cannabis a Schedule 1 drug while states continue to decriminalize it (2)
- Focusing on criminalization rather than harm reduction
- Restricting Access to Psilocybin and Other Psychedelics for Research
- [Add your personal favorite here]
Soon the FDA is (finally) going to take the axe to Sudafed PE (phenylephrine) a useless (3) (but highly lucrative) substitute for the decongestant Sudafed. This, of course, shines the spotlight yet again on the "war on meth," a quintessential example of our failed drug laws and policies. Here is a detailed Excel table that examines the risks and benefits of each drug. I hope it's not too technical.
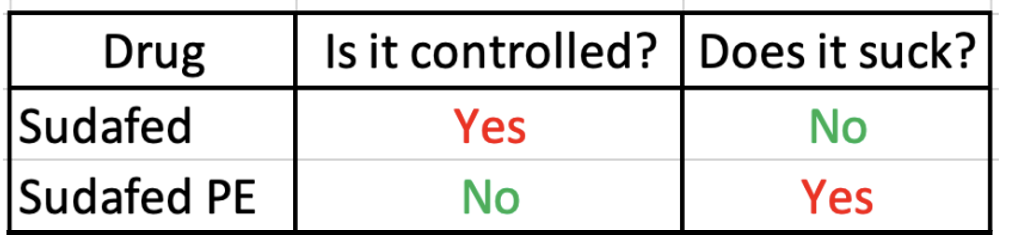
The meth mess, DEA style
As I have written before, thanks to the Combat Methamphetamine Epidemic Act (CMEA) (4) of 2006, Sudafed went from over-the-counter to behind it in a ridiculous attempt to address the "meth crisis." How did that work out? Not so well. The inability to get large quantities of Sudafed made things worse as chemists, using an easy workaround, started making a whole lot more of cheaper, purer meth. It's being made using the truckload by the superior P2P method.
How did that work out? About how you'd expect.
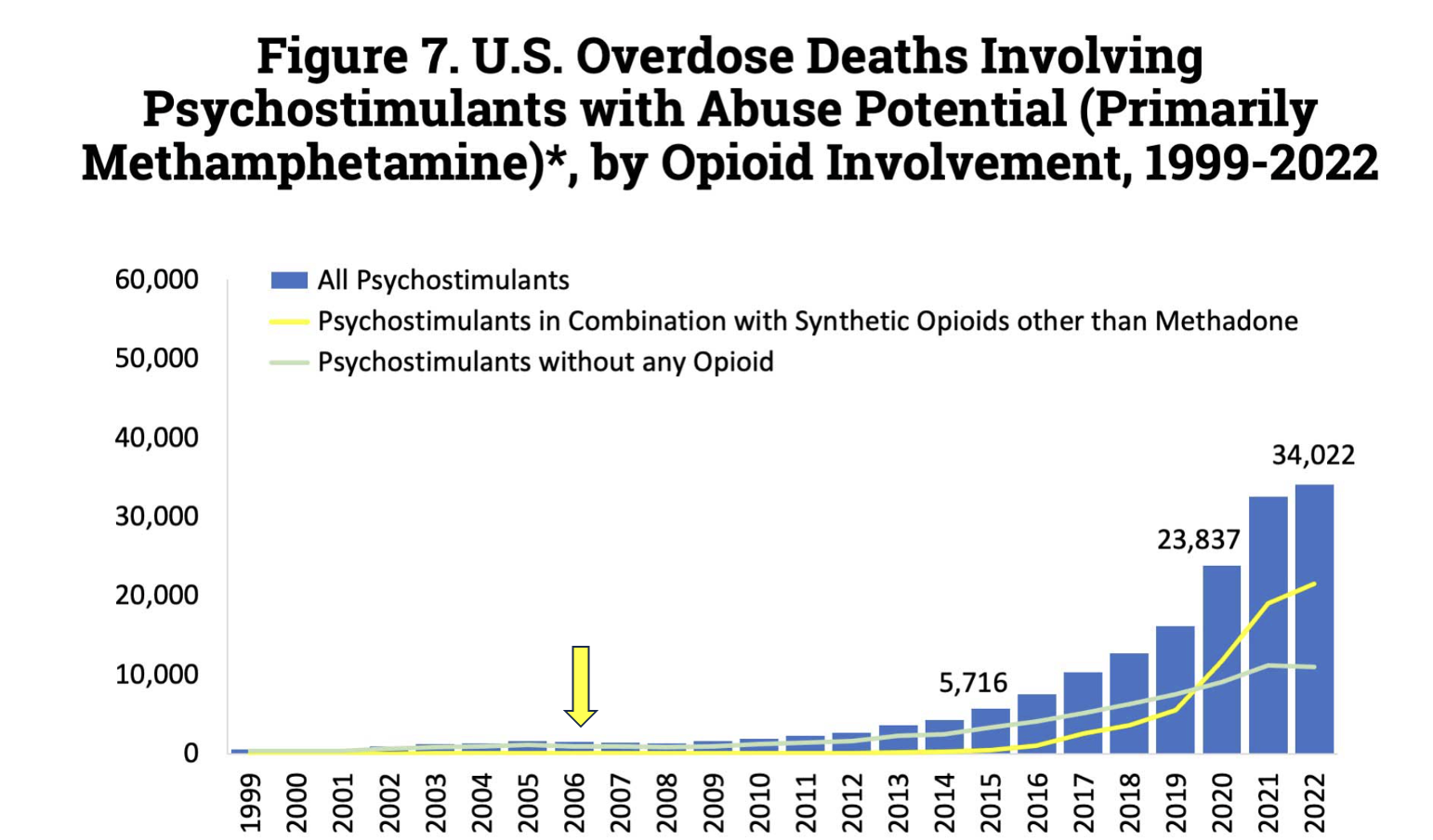
In 2006, when CMEA was passed, the number of fatal overdoses from methamphetamine was low, well under 2,000. This number ballooned to 34,000 by 2022 (not shown; that number rose to 36,000 in 2023). Graph: National Institute on Drug Abuse
The FDA dawdles
It's clear by now that phenylephrine (in oral form) has no business being sold over the counter (or behind, near, or in front of it). Given the lack of efficacy and side effects of the drug, it should have been a no-brainer for the FDA to take action to remove it from pharmacies. Wrong. As early as 2007 there were questions about the drug's efficacy and by 2009 a placebo-controlled study was published, concluding : "Phenylephrine was not significantly different from placebo in the primary [endpoint], mean change in nasal congestion score at more than 6 hours."
Yet, the FDA has still not acted. Much to the delight of Johnson & Johnson, no doubt.
Johnson & Johnson's very successful "placebo marketing plan"
It is difficult to come up with a whole lot of positives about phenylephrine. Here's one – it cannot be converted to methamphetamine. But neither can tomato soup, Ajax, or underarm deodorant. It would seem that the DEA doesn't care what you swallow as long as it has no potential for abuse or addiction.
But J&J cares. It has been selling a superbly profitable placebo – about $1.8 billion in 2023. Multiply that by 17 years and it can be roughly estimated that since 2006 consumers with stuffy noses sneezed a $29 billion donation at Johnson and Johnson.
But this amount pales by comparison to Tylenol, which, with few exceptions, does little or nothing for pain. Tylenol brand name sales are roughly $15 billion per year, meaning that in the past two decades, the company (5) has sold north of $300 billion of useless (or nearly useless) OTC pills.
Bottom line
Our drug policies and regulatory delays harm Americans medically and financially, allowing ineffective and costly medications to stay on shelves while depriving effective drugs to those who need them. Reforms to prioritize patient care, harm reduction over strict enforcement, and timely evidence-based decisions are long overdue.
NOTES:
(1) Bronx cheer is slang for a fart. Or in certain "cultures," a fart sound made by the mouth. As if it matters. You get the point.
(2) Continuing its insane policies, the DEA has indicated that it will hold hearings in 2025 to consider making cannabis a Schedule 3 drug, requiring a prescription. Why??
(3) Phenylephrine in oral form doesn't treat congestion, but it does so as a nasal spray. The drug has other uses, including shrinking hemorrhoids. Maybe the FDA got our ends mixed up. It happens.
(4) To be fair, the DEA didn't technically "make" the Combat Methamphetamine Epidemic Act (CMEA) of 2006. It was passed by Congress and signed by President George W. Bush. But the DEA, which was intricately involved in the writing of the law, pushed hard for its creation and passage.
(5) Technically, J&J no longer sells Tylenol. But don't be fooled. In 2023 Johnson & Johnson's consumer health division spun off it into a new company called Kenvue, which sells Tylenol (and other over-the-counter products). However, Johnson & Johnson retains a significant ownership stake in Kenvue.




