A recent New York Times article described how Mexican drug cartels were recruiting college students (actually posing as real recruiters) to make fentanyl. Why? Because the crackdown on fentanyl and its precursor chemicals from China was creating a shortage, forcing the cartels to take matters into their own hands.
What was left unsaid was the skill level of the students being employed: it's pretty bad, something I witnessed ad nauseam during four years as a teaching assistant in charge of the Organic 101 lab.
And we're not talking about some schlocky community college here. The University of Virginia is one of the hardest schools in the world to get into. And all the students in my sections were pre-meds, presumably the cream of the crop. Some of them had a knack for organic synthesis but most did not. After evaluating the quality of thousands of student-made samples over the years I can tell you that there can be a ginormous difference in the quality of what the students submit. For example...
Here's the first experiment I had to grade as a TA. It was the very simple conversion of aniline into acetanilide, a drug that was used years ago for pain control in horses and cattle (1). Here's the reaction.
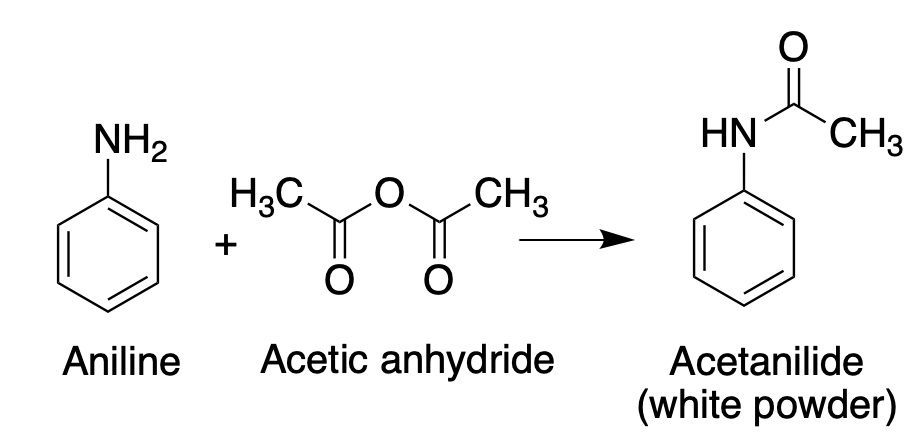
It's pretty hard to screw this reaction this up, but the UVA pre-meds gave it a shot anyhow. (Figure 1)

Figure 1. A (more or less) accurate portrayal of pre-med prepared acetanilide samples. From left to right: 1) This is how acetanilide is supposed to look; 2) Improperly purified acetanilide is purple; (Can you guess why?) 3) The texture of this snotball-looking thing isn't quite right; 4) No, I cannot explain how a miniature chicken head got into the vial.
Let's assume that the Mexican and UVA students have roughly the same level of lab skills. The quality of the fentanyl, which, despite being a rather easy task for a trained organic chemist, is going to be all over the place. (At this time I'm unaware of any Chicken Head Fentanyl being seized.)
Before we get into the chemistry, just for yuks let's imagine the differences in the interview process conducted by a chemical company and one by a cartel. It's a mixed bag.
Fentanyl precursors are way worse than acetanilide
Any idiot can convert 4-ANPP, the absolute best fentanyl precursor into fentanyl. It's that simple (Figure 2).

Figure 2. The reaction of 4-ANPP with propionic anhydride forms an amide bond. All peptides and proteins are composed of multiple amides. Amide formation is one of the fundamental reactions in organic chemistry. I have divided the fentanyl molecule into 4 non-arbitrary regions, A-D, each in a different color. The colors correspond to the precursor chemicals in Figure 3 below.
But, you can't buy 4-ANPP anymore (2) so it needs to be made. Which makes it necessary to show the following mess called Figure 3:
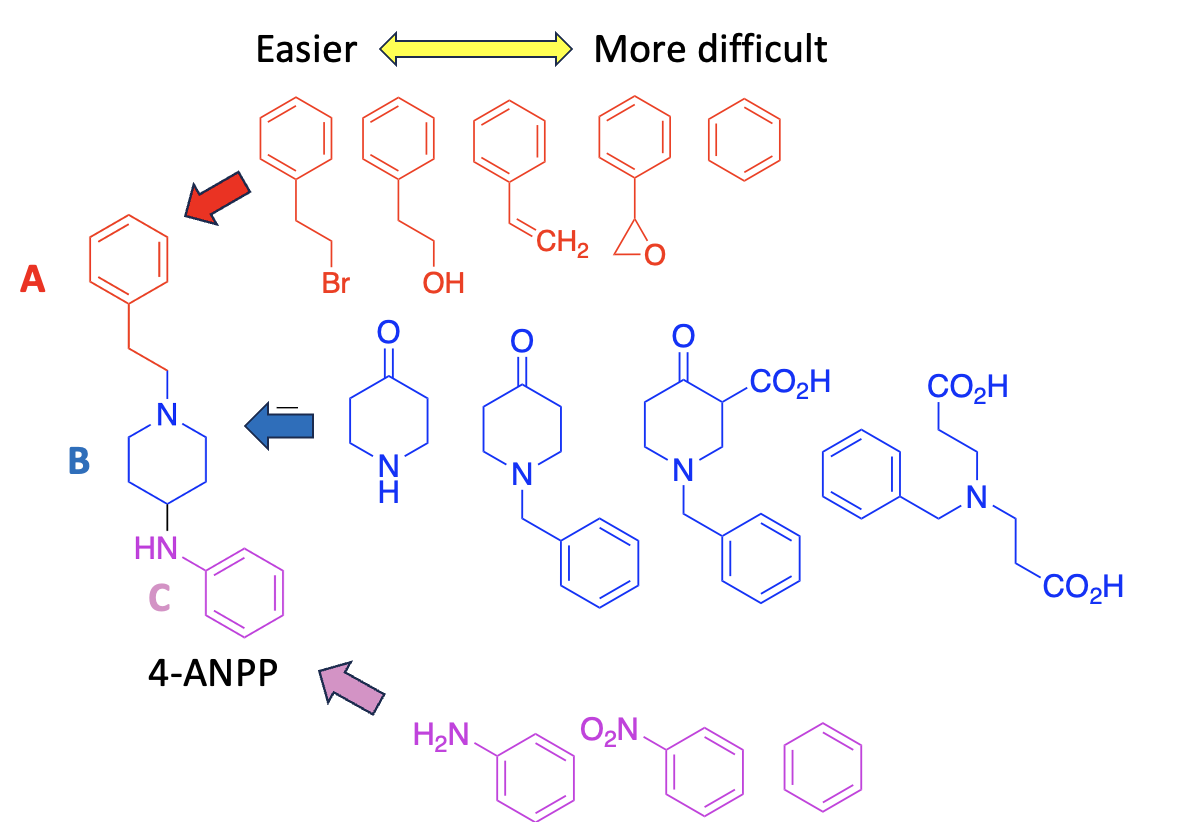
Mess called Figure 3. A retrosynthetic analysis of 4-ANPP, aka, how to make fentanyl when you don't have 4-ANPP available.
According to the Times article, this is where the cartel students are having problems. Where do they get the precursor chemicals? They need to make them from other precursor chemicals and this can be tricky.
The 5 chemicals in red represent some of the potential precursors for segment A. The red chemicals are arranged (approximately) so that the further they are from 4-ANPP in the figure the more effort is required to make them. For example, phenethyl bromide (left), if available, is easy to build directly into the fentanyl molecule while benzene (right) would require a number of steps, some of them a real pain in the ass, to do so.
Likewise, the chemicals in blue can all be used to form the B segment of fentanyl but going from left to right increases the difficulty, in this case by plenty. The same holds true for the magenta (C) segment. Any of these chemicals can be used to synthesize fentanyl but aniline (left) is the most convenient while benzene is the least.
What this article is about
Although I've tried to give you an idea of both the power and the strategies and complexities of organic synthesis, it's a mere outline of the process and one that non-chemists will not fully grasp.
Let's end with a look at some of the issues facing Mexican students who are "cooking" fentanyl.
- Organic synthesis is the art of converting one chemical to another.
- The fewer steps required the more efficient the route to fentanyl.
- Your average Org 101 student does not possess the skills to do this properly.
- Whatever fentanyl ends up on the street will be of unknown purity.
- There will be quite a range.
And what about working in a synthesis lab for a cartel vs. doing the same for US chemical company?
- The pay from the cartel will probably be better
- The lab conditions will be worse
- There will be no trained chemist to help with technical difficulties, just a guy with an AK-47.
- Although cartel workers will be spared the indignity of an annual performance review, poor performance will not be appreciated .
- In which case, the term "severance package" takes on a whole different meaning.
NOTES:
(1) Acetanilide use led to a condition called methemoglobinemia, where oxygen is not properly transported around the body. See: Methylene Blue: TikTok Craze, Plus a Dreaded Chemistry Lesson From Hell. It has been replaced by other NSAIDs and also Tylenol (groan). But Tylenol is toxic to cats as well as useless.
(2) Drug companies that manufacture fentanyl, an essential drug for analgesia and general anesthesia, are able to buy it from legitimate suppliers, but with strict regulations and record keeping.




