Due to increasing antibiotic resistance, microbiologists are on the lookout for unconventional ways to kill bacteria. Atypical methods range from phage therapy, in which bacteria-killing viruses are unleashed upon the microbes, to the use of "bed-of-nails" surfaces that physically rip bacteria apart.
Such out-of-the-box thinking was displayed yet again by a team of scientists from the University of Manitoba who created a hybrid antibiotic by tying together two different antibiotics with a molecular "rope."
One strategy to fight resistance is to hit a bacterium with multiple antibiotics at the same time. The reasoning is that, while it is relatively simple for a bacterium to spontaneously mutate to avoid the lethal effect of one antibiotic, it is very unlikely that multiple mutations will occur simultaneously that protect it against more than one. In other words, a bacterium may become resistant to antibiotic A or antibiotic B if they are used separately; but if used together, antibiotics A + B present a formidable challenge to resistance.
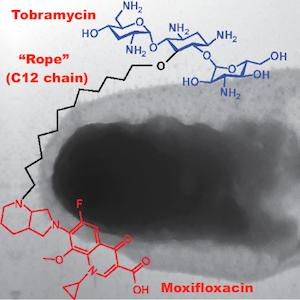
To create their hybrid, the researchers subjected the antibiotic tobramycin to a series of chemical reactions that (1) attached a molecular "rope" (a chain of 12 carbon atoms) to the center of the molecule and (2) tied the rope to a second antibiotic, moxifloxacin. (See diagram at the top of this article.)
Then, they tested how the hybrid antibiotic performed against clinically isolated samples of Pseudomonas aeruginosa, a bacterium that is both highly resistant to multiple antibiotics and is commonly transmitted in hospitals. (See below.)

Susceptibility to antibiotics is measured using the minimum inhibitory concentration (MIC, measured in micrograms per milliliter), which indicates the lowest amount of antibiotic necessary to prevent the bacteria from growing. (Thus, the higher the number, the more resistant the bacteria are to that antibiotic.)
Note that most clinical isolates of P. aeruginosa were highly resistant to both moxifloxacin (red box) and tobramycin (blue box) but that the hybrid antibiotic (purple box) was quite effective at inhibiting bacterial growth. The authors inferred that whatever molecular mechanisms allow these bacteria to be resistant to each antibiotic individually do not apply to the hybrid antibiotic.
So the team was curious to discover how exactly their hybrid antibiotic worked. Their investigation suggested that it operated in a completely different way than the original antibiotics. Instead of blocking protein synthesis (like tobramycin) or inhibiting DNA replication (like moxifloxacin), the hybrid exhibited an entirely new mode of action: It pokes holes in a bacterium's outer membrane and disrupts the electrical field potential (called the proton motive force) associated with the inner membrane. The former causes the bacterium to literally disintegrate (see below), and the latter disrupts various aspects of bacterial physiology.

Additionally, the hybrid antibiotic rescued moths infected with P. aeruginosa. Even better, when the authors tried to coax P. aeruginosa into becoming resistant to their hybrid antibiotic, they were unable to. This bizarre molecule, at least for the time being, is too much for this microbe to handle.
Source: Bala Kishan Gorityala et al. "Hybrid Antibiotic Overcomes Resistance in P. aeruginosa by Enhancing Outer Membrane Penetration and Reducing Efflux." J Med Chem 59 (18): 8441–8455. Published: 13-Aug-2016. DOI: 10.1021/acs.jmedchem.6b00867