Water (H2O) is essential to life and is ubiquitous; found even on planets, asteroids, and comets. It is a deceptively simple but very complex entity with many facets. Many people in developed countries consider access to quantities of drinkable (potable) and other water to be a given and at a low cost. The United Nations says that access to sufficient, safe water is a human right, but you get what you pay for. Access to universal assured safe drinking water has existed for only about 120 years with the development of microbiology to test for pathogens and engineering for filtration and disinfection water treatment.
Let’s begin a discussion about water and drinking water with some basics that not everyone knows.
- What is water, how much is there in and on the earth, and where is it?
- Is it all accessible to us?
- Is the total amount of water on the earth changing as populations and consumption increase?
- How does climate affect the availability and distribution of the earth’s water for our use and survival?
Just about everyone knows that water is chemically H2O; two hydrogens and one oxygen atom. Water is a small, simple molecule, but its unique properties make it so important to us for our existence. Those properties derive from its physical and chemical characteristics.
How much water is there on earth, where is it, and in what condition?
The water we access for drinking water and domestic and commercial uses typically cycles as rainwater. In a few remote areas, water is captured in cisterns. Rainwater becomes surface water in lakes, rivers, impoundments (an artificial lake), and groundwater. Surface waters are likely to have a few parts per million of natural organic components from vegetation and surface runoff. Some surface waters have some reused water, such as from treated wastewater. Much rain enters the ground, becoming groundwater. Groundwater can be deep or shallow wells, springs, and artesian wells. Groundwaters typically have lower amounts of natural organics, but they will have more minerals reflecting the geology in the area.
Technology can now allow the production of safe drinking water for planned and unplanned reuse, and desalination technology enables the conversion of saline waters into very low salinity drinking water that is virtually equivalent to distilled water.
Bottled waters can be from reprocessed municipal water, wells, springs, and artesian sources, and natural mineral waters are consumed as a supplement by some persons, especially in Europe.
- Earth has an estimated constant ~326 million trillion gallons (~1021 liters) of water, although its distribution varies
- ~96.5% is found in saline oceans, groundwaters, and bays
- ~1.75% is found in glaciers, ice caps (69% of freshwater)
- ~0.76% is fresh, not saline, groundwater (~30% of total freshwater)
- ~0.02% groundwater permafrost
- ~0.001% in the atmosphere
- ~0.008% in lakes and rivers
- Less than 1% of the Earth’s water is theoretically accessible as fresh ground and surface water using conventional technology, filtration, and disinfection.
- Less than 1% of delivered tap water is used in the home for drinking and cooking.
- About 70-75% of water is used for agricultural irrigation.
There is now generally available technology to convert millions of gallons per day of seawater and salty groundwaters to fresh drinking water using thermal or membrane desalination. Even sewage can be reliably and safely converted into potable water, as is being practiced at a large scale in several locations.
Desalination and sewage recycling are more costly than conventional technologies, but not excessively. In some Middle East countries, essentially all public drinking water at the “tap” is produced by desalination of seawater. There are numerous desalination plants in the USA, and the numbers and production volumes continue to increase. Drinking water is so cheap, about $4 per thousand gallons delivered to your tap, that we are spoiled when it comes to paying the cost. One reason for water shortages in some locations is that it is often subsidized, with consumers not paying the total cost of production.
The Water Cycle
The natural water cycle occurs through evaporation, rainfall, rivers flowing to the oceans, and percolation into the ground and aquifers. The quantity of water on earth has been considered to be essentially constant for millennia. When used for drinking, cooking, cooling, and industrial processes, it is returned to the environment, usually after treatment that produces better quality water than what was taken from the river. Using water does not reduce the total available quantity, and water not used productively ends up ultimately discharged to the ocean – along the way, it provides an aquatic ecosystem.
It is the nature of climate to change over time. Although the total water in the world remains constant, its location distribution will change over time. The Sahara Desert was a lush green environment due to monsoon rains after the ice age about 10,000 years ago. About 7000 years ago, the rains began to recede, and 5000 years ago, the Sahara became a desert. Today, population increases and shifts exacerbate problems caused by limited natural water availability in many locations. For example, Southern California has always had limited access to local natural water; as the population increased over the last 100 years, it has had to import water from the Owens Valley and by canals and pipelines for hundreds of miles from Northern California and the Colorado River.
Water is essential for just about everything.
Water, with its seemingly simple hydrogen and oxygen components, is essential and present in all living things; much of it is temporally chemically bound within fats, proteins, fibers, cells, and blood. The living organism is constantly taking in and discharging water, partly using it to produce, degrade and excrete essential chemicals. With death, we return to dust - these chemicals are decomposed by oxidative and reductive processes back to water and other components like carbon dioxide, amines, and nitrogen.
How did so much water get here?
Most of the water on earth was probably present in the bodies that collided to form the earth, and it is still here. Some water was and is being added by asteroids and meteors. A little might be lost through the stratosphere, but not much. The atmosphere consists mainly of oxygen (21%) and nitrogen(78%) with only traces of other gases and water vapor. The upper atmosphere, the stratosphere, is dry and cold, and it consists mainly of ozone, hydroxyl radicals, and nitric oxide formed by high-intensity solar radiation. Solar photolysis of methane at high altitudes produces some water vapor.
Physical Properties
Physically, water’s density its specific gravity is 1 gram/cc (1 gram/mL) - the basis used to measure the density of all materials. For example, the density of lead is 11.34 g/cc, so it is 11.34 times heavier than an equal volume of water. Water freezes at 32o Fahrenheit (00 Centigrade); its boiling point at normal pressure is 2120 Fahrenheit (1000 Centigrade).
As water freezes, its structure changes, and it becomes lighter, rising to the surface in your mixed drink or like an iceberg in the sea. Its maximum density is at 40C. As air temperature drops, as water (say in a pond) is in the process of freezing, its density decreases slightly, and this lighter, colder water on the surface freezes to solid ice at 0.919 g/cc. It takes a very cold winter for many water bodies to freeze solid because the surface ice insulates the water below from the cold air. That phenomenon alone is a significant factor in the functioning of our relatively moderate temperature environment as we know it.
Chemical Properties
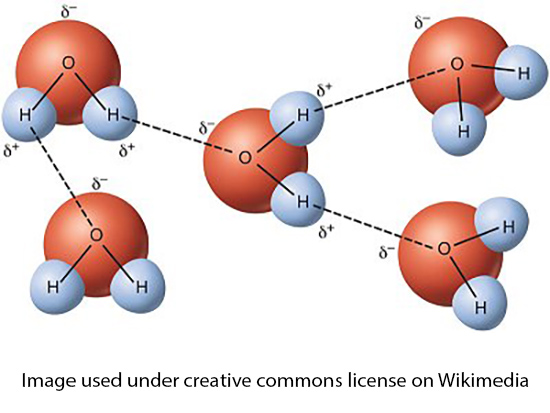 Water is polar with a more positive hydrogen side and a more negative oxygen side. That makes it a good solvent for charged ions and helps its electrical conductivity. The positive hydrogen side of the water molecule interacts with the negative oxygen side (hydrogen bonding) of other water molecules to act as a pseudo polymer - acting like a much heavier molecule than it actually is. Without that interaction, water would be a gas at ambient temperature, and there would be no lakes, rivers, or oceans.
Water is polar with a more positive hydrogen side and a more negative oxygen side. That makes it a good solvent for charged ions and helps its electrical conductivity. The positive hydrogen side of the water molecule interacts with the negative oxygen side (hydrogen bonding) of other water molecules to act as a pseudo polymer - acting like a much heavier molecule than it actually is. Without that interaction, water would be a gas at ambient temperature, and there would be no lakes, rivers, or oceans.
Most chemicals with molecular weights near water’s 18 Atomic Mass Units (AMU) are gases at normal temperatures and pressures. Ammonia (NH3), water’s nearest neighbor at 17 AMU, is a noxious, poisonous gas with a boiling point of -330C. Hydrogen sulfide, water’s next chemical neighbor, at 34, has a boiling point of only -59C. It takes more energy to volatize a heavier molecule. Still, water’s internal hydrogen bonding causes it to act as if its molecules are much heavier, preventing water from being all gaseous at “normal” temperatures, other than by evaporation.
Some basic water chemistry
A very common measured characteristic of water is its level of acidity or pH. Water (H2O) exists in equilibrium with a small amount of H+ and OH- ions. If the water is acidic, there will be more hydrogen than hydroxide ions. pH is measured in water as between 0 and 14. At neutral pH 7, both ions are in balance. When a strong acid, like HCl, is added to water, there are more H+ ions, and the pH can drop to 1 or less if sufficient acid is added; when a strong base is present in water (NaOH), the pH can rise to 14. Vinegar (about 5% acetic acid, a weak acid in water) has a pH of about 3.
This has been a broad and basic overview of the many aspects of water characteristics and science and providing it to consumers. Providing reliable quantities of safe and usable drinking water is a constantly increasing challenge that engages many disciplines.

