If you ask a chemist "What element with a one-letter symbol should really have a five-letter symbol instead?" many would say sulfur.
Its atomic symbol is S, but one could argue that "tink" should be tacked on to the "S" because many sulfur-containing chemicals are all kinds of nasty. Take a sniff at these...
- Methanethiol (aka methyl mercaptan) -1 of the 3 chemicals that give farts their lovely essence
- Hydrogen sulfide and dimethyl sulfide - The other 2
- Isoamyl mercaptan - One of the components expelled from a skunk's anal gland.
 OK, let's take a quick timeout to consider this matter. Even if your science education consists only of reading a single article on "Crazy Joe" Mercola's website, I think we can all agree that anything that comes spooging out of a skunk's anal gland isn't gonna smell so great.
OK, let's take a quick timeout to consider this matter. Even if your science education consists only of reading a single article on "Crazy Joe" Mercola's website, I think we can all agree that anything that comes spooging out of a skunk's anal gland isn't gonna smell so great.
- t-Butyl mercaptan - Added to natural gas to give it a smell (as a safety precaution because natural gas has no odor)
OK, you get it. Sulfur is stinky. Chemistry uber-blogger Derek Lowe makes his thoughts on the matter amusingly clear.
"Now, thiophenol (1) is not known as a great inducer of nostalgia. Like the other small-molecule sulfur compounds, it reeks without letup."
Derek Lowe, In the Pipeline, July 2004. Hilarious.
Smelly or not, sulfur plays a critical role in living organisms. One reason is that it has a split personality. Thiols (chemicals that contain a sulfur-hydrogen bond, also called mercaptans) are easily converted to disulfides, which can act to hold molecules together. But disulfides easily revert back to the thiols, which do not. This is one of nature's way of controlling the structure of proteins. This interconversion is also the reason that a perm works, that tires are stronger, and mucus is either thick or thin. It's all the same chemistry (Figure 1).

Figure 1. Two thiol molecules (left) are converted into one disulfide molecule by forming a sulfur-sulfur bond (an oxidation reaction). But the reaction is reversible; a disulfide can easily be converted back to its thiol components (a reduction reaction). R is short for any carbon atom.
DISULFIDES ARE SLEAZEBAGS
The reason that any of this stuff works is because disulfides absolutely LOVE thiols, and they don't much care which one they're going to hook up with. They will react with anything with an SH stuck to it (Figure 2.). It is this reaction that breaks the S-S bond.

Figure 2. Disulfides are not particularly discerning about their partners.
With certain exceptions...

Arguably, the most important sulfur-containing biomolecule is the amino acid cysteine, one of the 20 amino acids from which proteins are constructed, and one of only two that contain sulfur. Cysteine/cystine undergo the same interconversion (Figure 3). Since cysteine an amino acid it will be found in most proteins, so this interconversion plays a critical role in holding protein strands together or letting them move apart (Figure 6).

Figure 3. The interconversion of cysteine and cystine plays an enormous role in protein structure.
On a side note... Cysteine vs. cystine??? What evil sadist came up with this? Is it any wonder that organic chemistry keeps people out of medical school? Consider the case of Steve:

Poor Steve. He just couldn't keep his amino acids straight.
THE CHEMISTRY OF A PERM
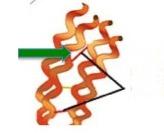
Keratins are a family of strong filamentous proteins that make up hair and fingernails. The strands are made even stronger because they are cross-linked to other strands via a disulfide bond between cysteine residues (2) on separate strands of proteins (Figure 4). This interconversion of cysteine and cystine is one of the reasons why thiols are so important for life processes. And for perms.

Figure 4. A model of keratin (orange curls). The red line (green arrow) represents the cystine disulfide bond that is broken.
Here are the perm steps. Some of you have experienced them and smelled them. This is how they work (Figure 5).
Step 1: Ammonium thioglycolate, a reducing agent, breaks the cystine disulfide bonds that interconnect keratin strands and gives hair its rigidity.

Figure 5. (Top) Ammonium thioglycolate (this is what stinks up the beauty parlor) chemically breaks the disulfide bond, forming two smaller keratin fragments, each having a thiol group instead of the disulfide.
Step 2: The "weakened" hair is then physically manipulated - curled, straightened, etc. to change its shape.
Step 3: Once this has been done (it takes some time to set) the hair is then rinsed with hydrogen peroxide, an oxidizing reagent which reforms disulfide bonds. But these new bonds will be in different from those in the original hair. The curling process moves the keratin strands around so that the disulfide bonds that form are different from the ones that were broken. It is this breaking, shifting, reforming process that gives the hair its new form (Figure 6).

Figure 6. The formation of new disulfide bonds gives hair its new shape.
HOW IS PERM CHEMISTRY RELATED TO CYSTIC FIBROSIS?
Strangely, the chemistry of mucus is conceptually identical to that of hair - the interchangeability of thiols and disulfides. But in this case, we want the disulfide bonds to be broken (reduced) but not reformed. In this way, thick mucus, one of the hallmarks of cystic fibrosis, becomes less thick. There is a drug called Mucomyst that does just this. Its chemical name is N-acetylcysteine (NAC) (3). When inhaled, N-acetylcysteine, just like ammonium thiolate did with hair, goes after disulfide bonds in the mucus (Figure 7).

Figure 7. The chemical structures of ammonium thioglycolate (top) and N-acetylcysteine (bottom). The "business end" of both molecules is the thiol group (blue circle).
The chemistry of mucus thinning is shown in Figure 8. Note the similarity to Figures 4 and 5.

Figure 8. Strands of mucoproteins, proteins which make up mucus, are linked together by disulfide bonds. This makes the mucus thicker and stickier. People with cystic fibrosis suffer from clogged airways because of built up thick mucus. When NAC is inhaled it breaks the disulfide bonds in mucus which causes it to become thinner. Then it can be coughed up or removed by suction. Source: Adapted from slideshare.net
Ain't chemistry great? The same reaction can style your hair or help you breathe better. The only difference between the treatment of hair and cystic fibrosis is that different thiols (which perform the same chemical transformation) are used and that (obviously) once the disulfide bonds in mucus are broken you would not want them to reform.
Pretty cool stuff. Although Steve may disagree.
NOTES:
(1) Thiophenol is a subgroup of thiols called aryl mercaptans. Sulfur is attached to a benzene ring. Doesn't matter. Still stinks.
(2) When an amino acid is part of a protein it is commonly referred to as a residue.
(3) If N-acetylcysteine sounds familiar it's because the drug is used to prevent liver damage caused by Tylenol overdoses. NAC is given orally or by IV, not inhaled. In this case, the mechanism of action is different. It has nothing to do with disulfide bonds.