The Frank R. Lautenberg Chemical Safety for the 21st Century Act amends the Toxic Substances Control Act (TSCA) and was signed into law June 22, 2016. It created a mandatory requirement for EPA to evaluate existing chemicals with clear and enforceable deadlines, to do so in a transparent fashion, and to do so using risk-based chemical assessments rather than rely on simple epidemiological correlations.
EPA selected the first 10 chemicals to undergo risk evaluation under the amended TSCA and to make those understandable for the public, the American Council on Science and Health is producing risk-based evaluations of each, which will then be compiled into a free downloadable book for consumers and policy makers.
What is Perchloroethylene?
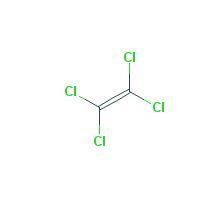
Perchloroethylene, a chlorinated hydrocarbon with the formula Cl2C=CCl2, is also known as PCE, PERC, tetrachloroethylene, tetrachloroethene, perchloro, and ethene, 1,1,2,2-tetrachloro. It is a nonflammable colorless liquid. It has a sharp, sweet, ether-like odor that most people can smell when it is present in air at concentrations as low as 1 part per million (ppm).
Perchloroethylene is a commonly used solvent (a substance, usually a liquid, capable of dissolving another substance). It is primarily used to produce fluorinated compounds, such as hydrofluorocarbons (HFCs) and hydrochlorofluorocarbons (HCFCs) followed by dry cleaning and vapor degreasing solvents. It is used as a starting material for making other chemicals and is used in some commercial and/or consumer products such as automotive care products, cleaning and furniture care products, lubricants and greases, adhesives and sealants, coatings, spot removers, typewriter correction fluid, shoe polish, printing inks, paint products, and household cleaners
Perchloroethylene breaks down very slowly in the air and so it can be transported long distances as it has been identified in atmospheric samples in remote locations such as Antarctica where no local sources of this substance exist. It evaporates quickly from water or soil into air, although some perchloroethylene may remain in the water or soil where it is slow to break down. It can also migrate through groundwater (or soil) into the air of homes and buildings through a process referred to as vapor intrusion.
Like many chlorinated hydrocarbons, perchloroethylene is a central nervous system depressant and can enter the body generally through respiratory or dermal exposure.
Why Is EPA Looking At This Under The Lautenberg Chemical Safety Act?
EPA scientists are currently looking at the likely routes of exposure to perchloroethylene in the environment and will be developing exposure scenarios, or pathways, of how the public comes into contact with perchloroethylene.
These exposure pathways will then be studied by EPA scientists by comparing the amount of perchloroethylene exposure in the pathway to its safe or virtually safe level (the virtually safe doses are derived from the cancer IURs and OSF discussed above). If human exposure in the pathway is at or below this safe or virtually safe level, then perchloroethylene exposure from the pathway is not considered to be a human health concern. If exposure is above this safe or virtually safe level, then the pathway might be considered as a possible health concern. Small excesses of the safe or virtually safe dose are seldom cause for concern since these safety levels are developed from conservative assumptions, including the use of safety factors that tend to exaggerate risk and exposure pathways that tend to exaggerate exposure.
Several pathways may be added together to suggest a health concern. In either event, regulations might be developed to lessen the exposure of perchloroethylene from this pathway(s). Alternatively, the ratio of a health benchmark to the human exposure, referred to as a Margin of Exposure (MOE), can be used as a threshold to determine the presence or absence of risk. To assess risks, the MOE estimate was interpreted as a risk of concern if the MOE estimate was less than the benchmark MOE (usually a 100) (see EPA, 2018). On the other hand, the MOE estimate indicated negligible concerns for adverse human health effects if the MOE estimate exceeded the benchmark MOE. Typically, the larger the MOE, the more unlikely it is that an adverse effect would occur.
See also EPA (2018) for specific questions related to the assessment of perchloroethylene under the new Lautenberg Chemical Safety Act (LCSA).
Controversy Over Perchloroethylene
Little controversy exists on the toxicity of perchloroethylene. Central nervous system (neurotoxicity), kidney, liver, development, and reproductive effects have been associated with its exposure in humans and animals. In general, neurological effects were found to be associated with lower perchloroethylene inhalation exposures. These neurotoxic effects have been analyzed in human controlled exposure, occupational exposure, and residential studies, as well as in experimental animal studies, providing firm evidence that they represent the principle, or critical, effect from perchloroethylene exposure (U.S. EPA, 2012a, 2012b).
EPA and other regulatory bodies have classified perchloroethylene as to its carcinogenicity to humans. The International Agency for Research on Cancer (IARC, 2013) has classified perchloroethylene as a Group 2A carcinogen, which means that it is probably carcinogenic to humans. EPA has classified perchloroethylene as likely to be carcinogenic in humans by all routes of exposure (EPA 2012a).
The U.S. National Toxicology Program (NTP) has classified perchloroethylene as reasonably anticipated to be a human carcinogen (NTP, 2014) and the American Conference of Governmental Industrial Hygienists (ACGIH, (2012) has classified perchloroethylene as an A3 carcinogen (confirmed animal carcinogen with unknown relevance to humans). All of these expert bodies agree that perchloroethylene is carcinogenic.
EPA (2018) is evaluating the pathways of exposure from industrial and commercial activities and uses of perchloroethylene and expects to include these in their risk evaluation. EPA (2018) also expects to evaluate the pathways of exposure resulting from consumer purchase and use products primarily intended for commercial use. The inhalation route has been identified as the primary route of exposure and EPA plans to further analyze inhalation exposures to perchloroethylene, including vapor intrusion and the risk towards bystanders. Given that the potential exists for dermal exposure to perchloroethylene from consumer uses, EPA expects to further analyze direct dermal contact with liquid perchloroethylene for consumers.
However, EPA considers the exposure of consumers to perchloroethylene via transfer of chemical from hand to mouth to be superseded by a combination of dermal absorption and volatilization. Therefore, EPA will not further evaluate this pathway. Nor does EPA expect that oral routes of exposure for mists or for incidental ingestion of perchloroethylene to have significant exposures. Thus, EPA will not include further analysis of these potential pathways. Neither will EPA further analyze exposure to consumers from disposal of consumer products.
EPA plans to evaluate perchloroethylene exposures to aquatic organisms from surface waters since aquatic and terrestrial species may be contaminated from environmental releases and waste streams associated with industrial and commercial activities. However, EPA will not further analyze the risk from rivers and streams, nor from air, drinking water, biosolids and disposal pathway exposures. This is because these pathways are under other environmental statutes administered by EPA, which adequately assess and effectively manage exposures and for which long-standing regulatory and analytical processes already exist. As one can expect, some of these exclusions may prove to be controversial.
Exposure To Perchloroethylene
Perchloroethylene is released into the environment following its use as a dry-cleaning agent, chemical intermediate, and metal degreasing agent. According to EPA (2012a, 2012b), workers and occupational non-users (workers who do not directly handle the chemical but perform work in an area where the chemical is used), consumers and bystanders (non-product users that are incidentally exposed to the product), and the general population are expected to be exposed to perchloroethylene primarily through inhalation, but also by skin and oral contact, such as through touching or ingesting contaminated surfaces and/or dust.
Workers in industrial and commercial settings are expected to have the highest exposures to perchloroethylene. Commercial and consumer users of the numerous products containing perchloroethylene can be exposed to perchloroethylene in indoor or outdoor environments. The general population is expected to have smaller exposures to perchloroethylene from industrial and/or commercial uses, from industrial releases to air, water or land, and from other conditions of use.
Perchloroethylene has been detected in most drinking water, groundwater, surface water and rainwater supplies (ATSDR, 2014). For example, ATSDR (2014) has reported the median value for indoor air in the United States, from 2,195 entries in the EPA’s database of volatile organic contaminants (VOC-AMBI), to be approximately 4.9 micrograms per cubic meter (μg/m3), with an average value of 20.7 μg/m3. In the drinking water of Cape Cod, Massachusetts, levels of perchloroethylene ranging from 1.5 to 7,750 microgram per liter (μg/L) have been reported, which was reduced to 40 μg/L after bleeding and flushing the drinking water pipes (ATSDR, 2014). Across the world, perchloroethylene has been measured in rivers at concentrations ranging from 0.01 to 168 μg/L, with levels in the U.S. ranging from 0.02 μg/L to 26.7 μg/L, but typically below 5 μg/L (EPA, 2018). In groundwater, perchloroethylene levels are usually below 10 μg/L, although levels as high as 1300 μg/L have been reported for contaminated sites due to unregulated or improper disposal of perchloroethylene (EPA, 2018).
Perchloroethylene is not likely to be in sediment based on its physical and chemical properties; for example, less than 5 μg/kg wet weight have been reported in the U.S.
Health Effects Of Perchloroethylene
Perchloroethylene is rapidly absorbed into the bloodstream following inhalation and oral exposures in both humans and animals. It can also be absorbed across the skin following dermal exposure to either pure or diluted solvent or vapors. Once absorbed, perchloroethylene is distributed to all tissues in humans and animals.
The highest concentrations of perchloroethylene are found in fat, but it can also reach high levels in both the brain and liver when compared to other tissues.
When human volunteers were repeatedly exposed to perchloroethylene by inhalation, it accumulated in the body. The absorbed perchloroethylene is mainly excreted in the breath as the parent molecule, particularly at higher exposures, but also metabolized in laboratory animals and in humans to a limited extent through at least two ways. The primary way results in non-toxic metabolites found in urine, but the other way results in the formation of relatively potent toxic metabolites (ATSDR, 2014, EPA, 2012a, 2012b). Differences in the metabolism of perchloroethylene among experimental animals and humans has been observed. For example, its rate of metabolism is faster in rodents than in humans (EPA, 2012a, 2012b), but it is less extensively metabolized compared to its closely related chemical, trichloroethylene.
Like exposure to any chemical, toxicity of perchloroethylene depends on the level to which one is exposed and the length of time of exposure. Both ATSDR (2014) and EPA (2012a, 2012b) have information on the health effects of perchloroethylene. Inhalation and oral exposures to perchloroethylene have resulted in damage to a number of tissues or organs in humans and animals. Effects observed in humans and animals after brief, high oral exposures appear to parallel those observed after inhalation exposures.
In both humans and animals, the primary targets following brief inhalation and oral exposure are the central nervous system, kidneys, and liver. While no data are available in humans regarding oral or dermal exposure to perchloroethylene following longer term exposures, long term inhalation exposures have caused adverse neurological effects. For example, humans occupationally exposed to perchloroethylene had irreversible central nervous system effects and toxicity to the kidney, liver, and immune system. Effects on color vision were also reported in some of these studies. Following prolonged (chronic) inhalation and oral exposures in animals, perchloroethylene also caused reduced survival and kidney effects (nephropathy or kidney damage) while inhalation exposure caused changes in the brain.
Both ATSDR (2014) and EPA (2012a, 2012b) also report reproductive effects from occupational exposure to perchloroethylene in both men and women. These effects including menstrual disorders, spontaneous abortion, sperm abnormalities, and decreased fertility. However, perchloroethylene levels in many of these studies were not measured and exposure to other solvents could not be ruled out. These limitations make it difficult to determine if there is an association between perchloroethylene inhalation and reproductive endpoints in humans.
Although the data in animals were limited, well-conducted studies suggest that perchloroethylene is a potential female reproductive toxicant. Effects caused in female animals included increased mortality, increased pre-and post-implantation loss, increased resorptions, and decreased body weight in the offspring.
Perchloroethylene and its closely related chemical, trichlorethylene, cause similar toxic effects. Epidemiological data provide evidence that exposure to perchloroethylene is associated with several cancer types such as non-Hodgkin lymphoma and multiple myeloma (cancers that form in different types of white blood cells) and bladder cancer, while more limited evidence suggests it is also associated with esophageal, kidney, lung, cervical and breast cancer (U.S. EPA, 2012e).
Studies in animals also provide evidence that exposure to perchloroethylene leads to an increased risk of developing cancer. In one species of animals, exposure to perchloroethylene has resulted in increased incidence and severity of mononuclear cell leukemia (an extremely common spontaneous disease of aging in this strain of rats) after inhalation exposure. In another species, both inhalation and oral exposures have resulted in increased incidences of liver tumors in both sexes (ATSDR, 2014; EPA, 2012a, 2012b).
Perchloroethylene Safe Levels
The federal and state governments develop regulations and recommendations to protect public health. Regulations and recommendations are often expressed as a safe or virtually safe level, that is, a level of a substance in air, water, soil, or food that is not expected to cause any adverse health effect, even in people who are sensitive to the chemical’s effects.
These safe levels are usually based on information from experiments with animals (usually rodents) at much higher levels of the chemicals than humans would typically encounter. The higher animal exposures are used to see what adverse health effects could occur. Scientists then conjecture what the adverse health effects could
possibly be in humans at a lower level of exposure. Scientists can then estimate the level that will likely protect humans. Sometimes these safe levels differ among federal and state organizations because they used different assumptions for human exposure, different animal studies, or employ methods that differ slightly. Other times these recommendations differ because new science develops that suggests different levels are toxic or safe. Recommendations and regulations are also updated periodically as more information becomes available.
ATSDR (2014) has derived safe levels for inhalation and oral routes only and are referred to as minimal risk levels (MRLs). MRL is an estimate of daily human exposure to a substance that is likely to be without an appreciable risk of adverse effects (non-carcinogenic) over a specified duration of exposure. MRLs can be derived for acute, intermediate, and chronic duration exposures for inhalation and oral routes.
ATSDR has derived a chronic (or lifetime) inhalation MRL of 0.006 ppm (0.03 mg/m3) for perchloroethylene based on color vision impairment in humans chronically exposed to perchloroethylene in the workplace. The chronic inhalation MRL was also used for shorter-term inhalation exposures because the blood concentration of perchloroethylene after acute- and intermediate-duration exposure is very similar to that after chronic exposure to the same concentration. ATSDR has also derived chronic oral MRL of 0.008 mg/kg/day. This MRL was derived based on the chronic inhalation MRL by estimating similar internal doses between these differing routes of exposure.
The chronic oral MRL was also used for shorter-term oral exposures MRLs. The World Health Organization (WHO) has established an air quality guideline value of 0.25 mg/m3 as an annual average (WHO, 2010) and a drinking water quality guideline value of 0.04 mg/L (WHO 2011). The long-term safe levels derived by EPA are referred to as the reference concentration or dose - RfC or RfD, an estimate of the chemical concentration or dose that will not cause non-carcinogenic effects during a specified exposure period - for the inhalation and oral routes, respectively. EPA (2012a) derived an RfC of 0.04 mg/m3 was based on neurotoxicity (reaction time, cognitive effects) in occupationally-exposed adults. An RfD of 0.006 mg/kg/day was based on the data from the inhalation study.
Although ATSDR discusses the potential of a chemical to be carcinogenic, it does not currently assess cancer potency or perform cancer risk assessments. In contrast, EPA has derived am Inhalation Unit Risk (IUR) - an estimate of the increased cancer risk from inhalation exposure to a concentration of 1 µg/m3 for a lifetime, which can be multiplied by an estimate of lifetime exposure (in µg/m3) to estimate the lifetime cancer risk - for the inhalation pathway and oral slope factor (OSF) - an estimate of the increased cancer risk from oral exposure to a dose of 1 mg/kg-day for a lifetime, which can be multiplied by an estimate of lifetime exposure (in mg/kg-day) to estimate the lifetime cancer risk - for the oral route. The EPA (2012) IUR for perchloroethylene is 1.8 × 10-3 per ppm (corresponding to 2.6 x 10-7 per microgram per cubic meter (μg/m3)) for liver tumors. The OSF of 2 × 10-3 per milligram per kilogram body weight [(2 x 10-3 (mg/kg-day)-1] was based on liver tumor data reported in the inhalation study.
More analyses in our series on the Lautenberg Chemical Safety Act compounds:
ACSH Explains: What's The Story On Pigment Violet 29?
ACSH Explains: What's The Story On N-Methylpyrrolidone?
ACSH Explains: What's The Story On Cyclic Aliphatic Bromides Cluster (HBCD)?
ACSH Explains: What's The Story On Carbon Tetrachloride?
ACSH Explains: What's The Story On Bromopropane?
ACSH Explains: What's The Story On Dioxane?
ACSH Explains: What's The Story On Trichloroethylene (TCE)?
ACSH Explains: What's The Story On Methylene Chloride (DCM)?
ACSH Explains: What's The Story On Asbestos?
REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists). 2012. Tetrachloroethylene. Threshold limit values for chemical substances and physical agents and biological exposure indices. Cincinnati, OH: American Conference of Governmental Industrial Hygienists, 55, 110. [Cited in ATSDR, 2014].
ATSDR (Agency for Toxic Substances and Disease Registry). 2014. Toxicological profile for tetrachloroethylene (Draft for public comment). Atlanta, GA: US Department of Health and Human Services, Public Health Service. Available at: http://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=265&tid=48
EPA (U.S. Environmental Protection Agency). (2012a). Toxicological review of tetrachloroethylene (Perchloroethylene). Washington, DC: U.S. Environmental Protection Agency. https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=106
EPA (U.S. Environmental Protection Agency). 2012b. Toxicological Review of Tetrachloroethylene (Perchloroethylene) (CAS No. 127-18-4): In Support of Summary Information on the Integrated Risk Information System (IRIS) (pp. 1077). (EPA/635/R-08/011F). Washington, DC. Available at: https://nepis.epa.gov/Exe/ZyPDF.cgi/P100DXJF.PDF?Dockey=P100DXJF.PDF
EPA (U.S. Environmental Protection Agency). 2018. Problem Formulation of the Risk Evaluation for Perchloroethylene (Ethene, 1,1,2,2-Tetrachloro). Office of Chemical Safety and Pollution Prevention. EPA Document# EPA-740-R1-7017. Available at: https://www.epa.gov/sites/production/files/2018-06/documents/perc_problem_formulation_5-31-2018v3.pdf
IARC (International Agency for Research on Cancer). 2013. Agents classified by the IARC monographs. Volumes 1–107. Lyon, France: International Agency for Research on Cancer. Available at: http://monographs.iarc.fr/ENG/Classification/index.php.
NLM (U.S. National Library of Medicine). 2018. ChemIDplus: Tetrachloroethylene. Toxnet database. Available at: https://chem.nlm.nih.gov/chemidplus/rn/127-18-4
NTP (U.S. National Toxicology Program). (2014). Tetrachloroethylene (CAS No. 127-18-4). Report on carcinogens. Fourteenth edition. Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service. Available at: https://ntp.niehs.nih.gov/ntp/roc/content/profiles/tetrachloroethylene.pdf
WHO. 2010. Guidelines for indoor air quality: Selected pollutants. Geneva, Switzerland, World Health World Health Organization. See: http://www.euro.who.int/__data/assets/pdf_file/0009/128169/e94535.pdf.
WHO. 2011. Guidelines for drinking-water quality. 4th ed. Geneva, Switzerland, World Health Organization. See: http://www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/index.html.
Data provided by
Michael Dourson, PhD, DABT, FATS, FSRA
Bernard Gadagbui, MS, PhD, DABT, ERT
Bethany Hansen, MA
all of Toxicology Excellence for Risk Assessment (TERA)