
If you are driving around the southwest and find yourself in the middle of nowhere...
...pay attention. Because you never know when you might be near a place of historic significance. In this case, there's even a road sign to help find the place...

Road sign for Chloride, AZ - a historic mining town in the middle of nowhere.
How could a chemist possibly pass up the chance to go to Chloride, Arizona? Especially while on a road trip of old Rt. 66. I could not. Hence, the detour.

The road to Chloride. Not much there. And that Pop. 352 figure seemed to be off by about 351.
There wasn't much there – a ghost town that the ghosts abandoned – but I did learn something interesting. What about the name? Why would anyone bother to form a town in 1860 in the middle of nowhere just to mine salt? Because it's not sodium chloride that gave the town its name. It was named after silver chloride and there just happened to be a bunch of it in the ground back then. Silver chloride doesn't look especially interesting, but its chemistry is.

Silver chloride. Photo: Wikipedia
Now that I've suckered you all in with these cool photos you must pay the price. And that price is...

Yessiree folks! It's time for The Dreaded Chemistry Lesson From Hell® And today we're going to learn about (what else) silver chloride.
THINGS YOU DON'T WANT TO LEARN ABOUT SILVER CHLORIDE BUT ARE GONNA ANYHOW:
- Silver chloride is a white solid which is extremely insoluble in water. That can be easily demonstrated by the following experiment (Figure 1):

Figure 1. Silver nitrate (beaker) is very water-soluble. So is sodium chloride (test tube). But when you mix them, silver chloride (the white "cloud") forms immediately and then precipitates to the bottom of the beaker. It can be collected by filtration. (Photo: Google sites)
This insolubility can come in handy. If you add tap water to a solution of silver nitrate it will almost always turn cloudy. The cloudiness results from the reaction of the silver nitrate with chloride in your drinking water, the more chloride the cloudier it turns. In fact, silver nitrate can be used to quantitate the amount of chloride in a water sample. This is also why chemists use distilled water when working with silver nitrate.
- Silver chloride is sensitive to light.
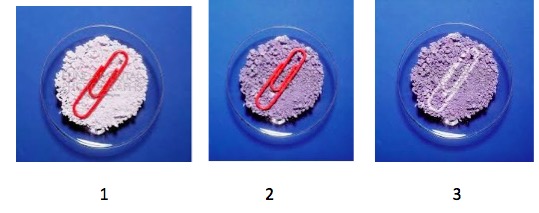
Figure 2. Photosensitivity of silver chloride. (1) "Fresh" silver chloride partially covered by a paper clip. (2) With time the white changes to light purple. (3) The paper clip is removed. Photos: Fundamental Photographs
The color change when silver chloride is exposed to light (Figure 2) can be explained by simple chemistry. Light reacts (slowly) with silver chloride, decomposing it to silver metal (1) and chlorine. This reaction was the basis of traditional photography.
- Although silver chloride won't dissolve in water it's amazing what a little ammonia will do
If you take the beaker in Figure 1, even if there is so much chloride added that looks like a wet solid, and add some plain old stinky ammonia all of the white solid will be dissolved and the beaker will contain a solution the is indistinguishable from water. Ammonia does the job because it forms a water-soluble complex with silver+1. Here's the reaction:
AgCl (solid) + 2 NH3 ---------> Ag(NH3)2+ Cl- (water-soluble)
- Pure silver can be recovered from silver chloride using this chemistry
Once its solubilized (the silver chloride reacted with ammonia), ionic silver is easily converted to metallic (atomic) silver by using a reducing agent - a chemical that donates electrons. There are many of these; one that works well is sodium dithionite. Here's the reaction.
Na2S2O4 + 2[Ag(NH3)2+ Cl-] → 2Ag0 (metal) + 2SO2 (gas) + 2NaCl
OK, The Dreaded Chemistry Lesson From Hell® is over. You are free to go about your day. After reading this you are probably ready to go on a vacation. If you want some tips on where to eat should you happen to be in the vicinity of Chloride feel free to ask.
But you better keep your eye on the speed limit. They also have this in Chloride.

NOTE:
(1) The purple color can be explained by the minuscule size of the silver particles. When you shake up silver nanoparticles with water you get colloidal silver, a quack remedy that has been used for a century. Despite the lack of any evidence of its safety or efficacy people still take it, perhaps because they think it's organic (it's not). Swallow a bunch and go out in the sun and you could very well develop argyria, like this dope below, who took the stuff as an "alternative therapy" for 40 years.

Photo: The Today Show


