
Aging is a complex process involving many variables interacting in linear and nonlinear ways closely linked with nearly all diseases. Understanding aging’s molecular changes is critical to increasing the health span. Although many studies have focused on linear, 1 for 1 changes during aging, evidence shows that the risk of aging-related diseases and mortality increases nonlinearly over time. Aging is not a simple linear process. The recent use of the term “clocks,” applied to biological functions while measuring molecular changes, often provides a single, oversimplified measure, inadequate to describing aging other than predicting the difference in one’s biological versus chronological age.
High-throughput “omics” technologies, characterizing and quantifying pools or groupings of molecules, have allowed researchers to study molecular changes at a system level, revealing unique aging trajectories across different organs. These studies have shown that the susceptibility and resilience to aging-related diseases vary significantly between organs, systems, and individuals.
“Omic modeling” is minimally invasive, relying on a small blood sample, and can predict mortality, organ-specific functional decline, disease risk, progression, and aging differences between tissues. It is a tool that can be applied to assess the effects of health interventions, like lifestyle changes and drug therapies. Consider three studies.
“Collectively, organ age gap associations with disease and blood biochemistry demonstrate that aging models derived from organ-specific plasma proteins capture disease-relevant heterogeneity of aging within and across individuals, which is not captured by other aging clocks or clinical markers.”
In the first study, using measured levels of human blood plasma proteins from specific organs and machine learning models, researchers analyzed aging in 11 major organs in five independent cohorts involving 5,676 adults across various ages. Estimating organ age by comparing protein patterns to chronologic age, they found that
- individuals with the same conventional age gap had diverse organ aging profiles.
- Although it might be expected that extreme aging in one organ would co-occur with extreme aging in other organs, the majority of variance in one organ age gap is not explained by others. Roughly 1.7% of individuals showed extreme aging in multiple organs. Approximately 18.4% of individuals had a highly organ-specific e-ageotype dominated by aging only one organ. [1]
- Accelerated organ aging increases organ-specific diseases and mortality risk
- For some organs, there was a nonlinear relationship between the age gap and mortality risk
In the second study, researchers developed a proteomic age clock using blood proteomic and mortality data from a large sample of 45,441 participants in the UK Biobank, gathered over 11 to 16 years. The model was subsequently “validated” in 3,977 participants from China’s Kadoorie Biobank and 1,990 participants from Finland’s FinnGen, making their findings,
“highly generalizable across populations of diverse genetic ancestry and diverse levels of morbidity.”
The study examined the impact of the difference between protein-predicted age and chronological age, the proteomic age gap (ProtAgeGap) on 27 aging-related phenotypes, all-cause mortality, and the incidence of 26 common age-related diseases. [2]
The research identified multiple aging-related proteins, including those impacting
“ (1) cell adhesion and extracellular matrix (ECM) interactions (2) immune response and inflammation (3) hormone regulation and (5) protease activity and enzymatic function (6) regulation of body weight and energy balance (7) neuronal structure and function and (8) development and differentiation.”
Increasing ProtAgeGap was associated with
- Abnormal biomarkers of kidney, liver, metabolic, and immune dysfunction
- Poor self-rated health, slow walking pace, self-rating older appearance, sleeping 10+ hours per day, feeling tired daily, and frequent insomnia.
- Higher frailty index, blood pressure (systolic and diastolic), reaction time, arterial stiffness, BMI, lower bone mineral density, fluid intelligence, lung function, and hand grip strength.
- Increasing multimorbidity
Proteomic aging was a strong predictor of diseases, showing different age-specific incidence rates of all-cause mortality and 14 common noncancer diseases. When adjusted for lifestyle considerations, i.e., smoking and eating, ProtAgeGap “remained significantly associated” with mortality and all noncancer outcomes for never-smokers and those individuals within a normal weight range measured by BMI.
The final study, published last week, conducted longitudinal multi-omics profiling on a cohort of 108 individuals aged 25 to 75, with some monitored for up to 6.8 years. Researchers collected diverse biological data, including transcriptomics, proteomics, metabolomics, and various microbiome samples, totaling 5,405 samples, adjusting for factors like BMI, sex, and ethnicity, to gain insights into the biological shifts that occur with age.
Most molecules and microbes, 81%, demonstrated nonlinear changes with age; few, 6.6%, changed linearly over time. The research identified 11 clusters changing during aging, and three of them “displayed compelling, straightforward, and easily understandable patterns that spanned the entire lifespan.” The three pathways are critical in genomic stability, gene expression, and metabolic balances.
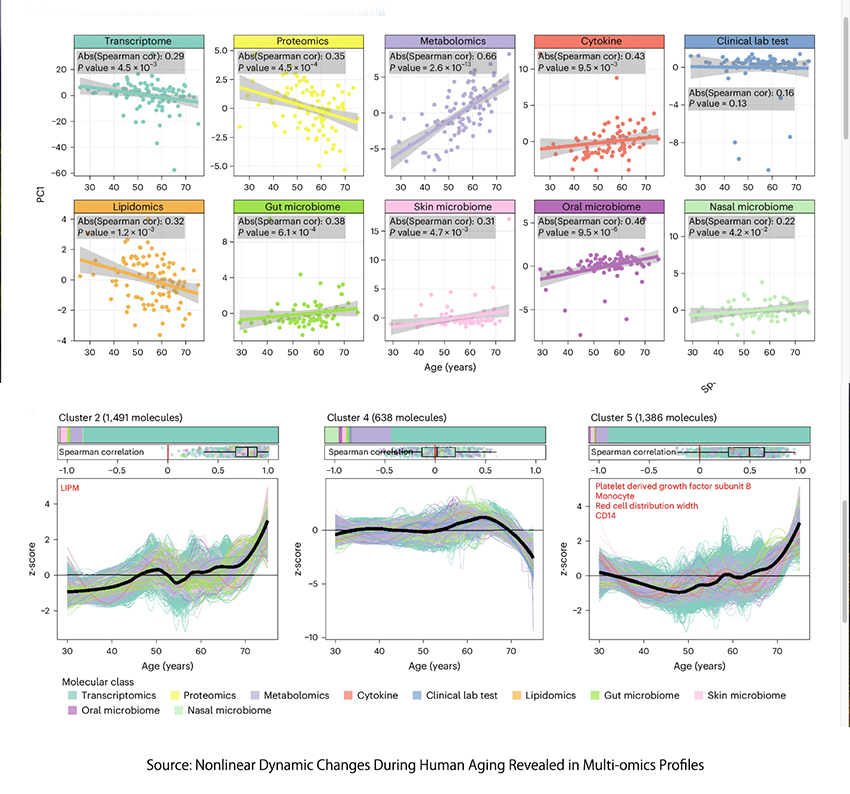
- In cluster 2, pathways such as phenylalanine metabolism and markers like plasma glucose show a gradual increase in risk, which accelerates after age 60. This suggests a nonlinear escalation in the risk of cardiovascular disease, kidney dysfunction, and type 2 diabetes as people age.
- Cluster 4 highlights a nonlinear decrease in the ability to metabolize caffeine and the immune regulatory role of the cytokine MCP1, supporting the idea of increasing disease risk as individuals grow older.
- Cluster 5 included tests indicating a nonlinear decline in the oxygen-carrying capacity of blood.
- The changing patterns resulted in “significant dysregulation occurring at two major periods occurring at approximately 44 and 60 years of chronological age.”
“The identified crests represent notable milestones in the aging process and suggest specific age ranges where substantial molecular alterations occur.”
In those “crests” of dysregulation, in our middle years, there was dysregulation of platelets degranulation, compliment, and coagulation cascades, which impact cardiovascular disease, blood fluidity, and thrombotic events. And dysregulation of components of the extracellular matrix involved in our skin and muscle’s structural stability, elasticity, and strength. It should not be surprising that the incidence of cardiovascular disease and a cash flow for Botox, fillers, and lifts to cosmetic surgeons rises during these aging milestones.
The complexities of aging extend far beyond the markers of time and physical appearance. As emerging research highlights, the aging process is a dynamic interplay of molecular changes that do not adhere to a simple, chronologic progression — each organ, system, and individual ages uniquely, with susceptibility to age-related diseases varying significantly. The application of "omics" has uncovered intricate patterns, offering a more nuanced understanding of how we age. This evolving knowledge challenges traditional models of aging.
[1] The study found that specific organ e-ageotypes are strongly associated with particular health conditions: the kidney’s e-ageotype is linked with metabolic diseases like diabetes, obesity, hypercholesterolemia, and hypertension; the heart’s associated with heart diseases such as atrial fibrillation and heart attacks; the muscle’s connected to gait impairment. Interestingly, it was the overall e-ageotype that was most significantly associated with Alzheimer's disease (AD)
[2] Associations included a comprehensive frailty index, 16 individual measures of physical, e.g., slow walking pace and grip strength and cognitive function, e.g., reaction time and fluid intelligence); and ten measures of biological aging, e.g., telomere length and clinical blood biochemistry, e.g., albumin and creatinine.
Source: Organ aging signatures in the plasma proteome track health and disease Nature DOI: 10.1038/s41586-023-06802-1
Proteomic aging clock predicts mortality and risk of common age-related diseases in diverse populations Nature Medicine DOI: 10.1038/s41591-024-03164-7
Nonlinear Dynamics Of Multi-Omics Profiles During Human Aging Nature Aging DOI: 10.1038/s43587-024-00692-2


