It's more than a little disturbing that I have to be writing about this, but in light of Vladimir Putin's most recent threat to use nuclear weapons, it's a safe bet that bottles of potassium iodide tablets are either flying off shelves, especially in Europe or will be soon.
This is because the tablets are effective in preventing cancer from nuclear radiation, but only one type: thyroid cancer. Examining some of the properties of the element iodine explains why.
Formation of radioactive iodine-131
During nuclear fission – the process used in nuclear plants and bombs – radioactive isotopes of uranium and plutonium decay to form dozens of elements, some radioactive and some not. One is iodine-131, a radioisotope of iodine-127 (aka, "normal iodine"). I-131 decays, giving off beta particles and gamma rays, both radioactive and carcinogenic (1). (Nuclear chemistry is complex and difficult to grasp, and it is beyond the scope of this article.)
Here's a simplified version of the fission process (2,3):
First step - the explosion
235U + neutron →131I + multiple fission products + much energy
Second Step - fallout
131I decay ----> 131Xe (stable) + beta particles + gamma rays
Why is the thyroid gland the target for radioactive iodine?
Roughly three-quarters of the iodine exists in the thyroid gland with smaller amounts found in the blood, kidneys, and mammary glands. The thyroid contains two biologically important (4) thyroxines called T3 and T4 :
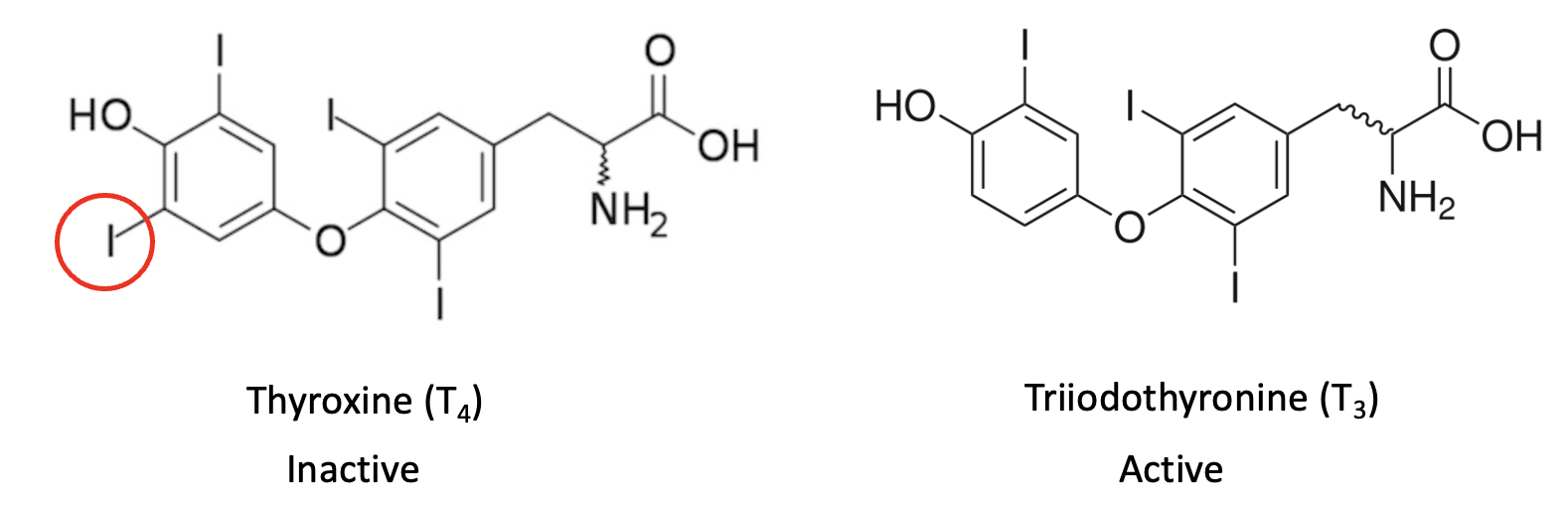
Figure 1. The two primary thyroid hormones. Most of the iodine in the body is stored in the thyroid gland in the inactive T4 form, which is converted (reversibly) to T3 by a complex feedback process. Chemically, the only difference between the two is iodine (red circle) in T4 replaced by a hydrogen in T3.
Why is this important? The chemical structures above are not just for show. T3 and T4 are interconvertible through an enzymatic process, during which iodine is both released and absorbed. Because I-131 is chemically identical to stable I-127, it enters the body's "iodine pool" and is eventually stored as radioactive T4 in the thyroid gland. There, its radiation bathes thyroid cells, damaging DNA and increasing cancer risk. This explains why I-131 is such a threat and why potassium iodide is so effective at preventing thyroid cancer. By saturating the thyroid with stable iodine, potassium iodide blocks the uptake of radioactive iodine. In fact, a single tablet contains 100,000 to 1 million times the amount of iodine typically absorbed during a nuclear event, effectively preventing radioactive iodine from concentrating in the thyroid.
Why is potassium iodide not effective against other cancers?
Having gotten this far, it should be obvious that potassium iodide prevents thyroid cancer through a specific process. This is why it is not effective against any other cancer. If iodine-131 was the only product of a nuclear event it would be possible to prevent virtually all radiation-induced cancers. However, this is far from the case. Here is a short list of some of the other dangerous radioisotopes formed in a nuclear explosion:
- Cesium-137 - Long half-life (>300 years). Emits beta particles and gamma radiation throughout the body, causing cancer, tissue damage, and radiation poisoning
- Strontium-90 - Long half-life (28 years). Strontium is chemically similar to calcium. It accumulates in bones and teeth causing leukemia and bone cancer.
- Tritium (3H) - Long half-life (12 years). Is converted to 3H2O, which is (almost) chemically identical to water. It gets incorporated everywhere that water does. Causes DNA damage. cancer.
It should be noted that, unlike the dangerous radioisotopes above, I-131 has a much shorter half-life (~8 days). In 35 days, 95% of it will be gone.
Bottom line
Although potassium iodide is very effective in preventing the uptake of iodine-131 and thyroid cancer it is not a panacea. Nor is it for everyone. According to the CDC, it is recommended only for people under 40 and people who are pregnant or breastfeeding because it can have harmful effects, such as thyroid inflammation and dysfunction.
While potassium iodide can prevent thyroid cancer, it underscores the importance of avoiding nuclear warfare altogether—the only true way to protect humanity from such catastrophic consequences.
Notes:
(1) Beta particles damage nearby tissues, while gamma rays penetrate further and cause systemic effects.
(2) Although I have shown the fission reaction of uranium, plutonium-239 is preferred for nuclear weapons. The bomb is smaller. The nuclear reaction is similar. Both form I-131 in the explosion.
(3) ChatGPT: "Fission is a nuclear process in which a heavy atomic nucleus (like uranium-235 or plutonium-239) splits into two smaller nuclei, releasing a large amount of energy, neutrons, and sometimes gamma radiation. This process can occur naturally or be induced by a neutron, and it powers both nuclear reactors and atomic bombs."
(4) Other T's with fewer iodine atoms are present in small quantities. They are not relevant to this article.




