
Sometimes drugs behave very well. They do what they are supposed to so, and do it well, maybe even without side effects (1). We are fortunate to have one of these that works against a very common infection—herpes simplex virus (HSV).
There are two versions of the virus. HSV-1 is the type that causes cold sores, and HSV-2, which causes genital herpes (2). The two viral types are similar enough that they both respond well to the same drug—acyclovir (3). The way that acyclovir works is a textbook example of an antiviral drug in action—shutting down an essential step that brings viral replication to a halt. The essential step that is shut down is the prevention of the formation of a phosphate ester bond—a key step in the manufacture of viral DNA. No DNA, no more viruses. Pretty cool stuff.
Acyclovir inhibits DNA polymerase—the enzyme that is responsible for assembling the individual components of DNA into a very large chain, DNA itself. The drug is classified as a chain terminator, for reasons that will become apparent later on.
On the left is the structure of guanine (G, in the blue circle)—one of the four bases that exist in DNA—bound to deoxyribose (green circle), the sugar that makes up DNA (the D stands for deoxy—lacking oxygen) (4). On the right is the structure of acyclovir. The similarity (blue) of the two structures is evident, but so are the differences (green). The difference between the two is shown in red; 1t is obvious that acyclovir contains only a small portion of ribose.
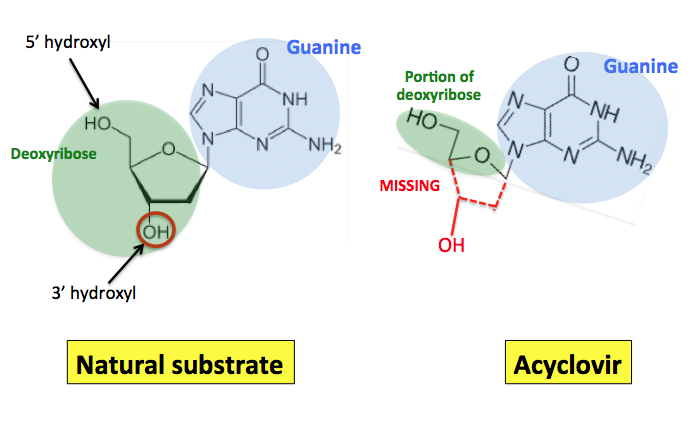
Most importantly, the 3’ hydroxyl group (red circle)—the place where the next building block would normally be attached—isn’t present in acyclovir. This is why acyclovir works, and this becomes rather easy to see when you examine the normal extension reaction compared to what happens when acyclovir gets into the mix.
The enzyme that promotes (catalyzes) the phosphorylations that assemble the DNA chain is called DNA polymerase. It grabs nucleotides that are floating around, holds them in the correct position so that they sit near the growing DNA chain, and chemically attaches the new nucleotide to the growing chain, extending the chain by one unit each time. But it has an Achilles heel: It cannot distinguish between guanine-deoxyribose and acyclovir. So, when an acyclovir molecule comes calling, the enzyme happily invites it in.
If enzymes had the capacity to experience regret, DNA polymerase would do just that at this point. Because once it incorporates acyclovir into the growing DNA chain, it is toast. It can no longer do its job. Note the difference between when 1) Normal elongation: another base is attached to the 3' hydroxyl group (left), which results in the addition of one more piece being added to the growing chain, and 2) Chain termination (right), when there is no place for the next piece to be attached, so the chain cannot grow. The new bond that is formed is represented by an orange dashed line, and the new base is inside the orange circle.

The mechanism by which acyclovir works is both elegant and simple. It also provides an opportunity to see on a molecular level how and why a very good drug works.
Like I said, pretty cool stuff.
Notes:
(1) Of course, all drugs have side effects. These are a function of the inherent toxicity of the drug and the dose. Acyclovir is usually very well tolerated.
(2) It’s not quite that simple. For a more comprehensive discussion of the differences between HSV-1 and HSV-2, see “A Herpes Vaccine Erupts in the News.”
(3) Acyclovir is now rarely used. Instead, Valtrex (valacyclovir)— a pro-drug of acyclovir is preferred because it is better absorbed, and can be dosed once or twice per day, instead of 4-5 times. This is because acyclovir is poorly absorbed in the gut. But Valtrex is adsorbed in the small intestine by an active-transport mechanism. Once in the bloodstream it breaks down rapidly to give acyclovir, which will then have a much higher level in the blood.
(4) When any of the bases (A,C,G,T) is bound to deoxyribose, this is called a nucleoside. When it also contains phosphate groups, it is called a nucleotide.



